Structure of Atoms
Get insights from 148 questions on Structure of Atoms, answered by students, alumni, and experts. You may also ask and answer any question you like about Structure of Atoms
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
(i) One mole of methane (CH4) has = 6.022 * 1023 molecules
No. of electrons present in one molecule of CH4 = 6 + (4 x 1) = 10
No. of electrons present in 6.022 * 1023 molecules of CH4 = 6.022 * 1023 * 10
= 6.022 * 1024 electrons
(ii) (a)
Calculation of total number of carbon atoms
Gram atomic mass of carbon (C-14) = 14 g = 14 * 103 mg
14 g or 14 * 103 mg of carbon (C-14) has 6.022 * 1023 ato
New answer posted
6 months agoContributor-Level 10
(i) Mass of an electron = 9.10939*10−31 kg = 9.1 * 10-28 g
⇒ No. of electrons in 9.1 * 10-28 g = 1 electron
Therefore, No. of electrons in 1 g = 1/9.1 * 10-28 = 1.098*1027
(ii) One mole of electrons = 6.022 * 1023 electrons
Mass of 1 electron = 9.1 * 10-31 kg
Mass of 6.022 * 1023 electrons = (9.1 * 10-31kg) * (6.022 * 1023) = 5.48 * 10-7 kg
Charge on one electron = 1.602 * 10-19 C
Charge on one mole electrons = 1.602 * 10-19 * 6.022 * 1023 = 9
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: option (iii)
According to Heisenberg's uncertainty principle it is impossible to determine simultaneously, the exact position and exact momentum (or velocity) of an electron. Thus, it implies that determining the trajectory of
an electron is impossible as it requires exact position and velocity which is not possible as per the uncertainty principle.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: option (ii)
The black body is the ideal body that absorbs and emits radiation of all frequencies. The radiation that it emits is said to be black body radiation. With an increase of temperature, the frequency of the black body radiation increases.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: option (i)
As the chemical properties depend upon the number of electrons present in the atom thus the isotopes resemble the same chemical properties as they have the same number of electrons i.e. atomic number.
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
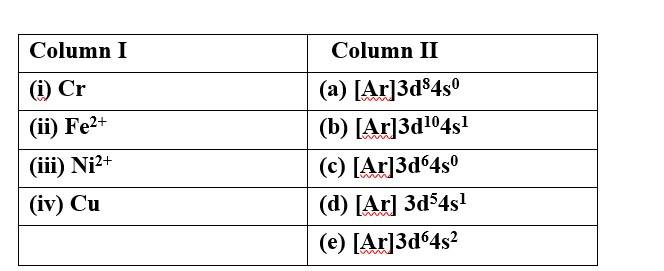
(i) Cr (d) [Ar] 3d54s2
(ii) Fe2+ (c) [Ar]3d64s0
(iii)Ni2+ (a) [Ar]3d84s0
(iv)Cu (b) [Ar]3d104s1
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
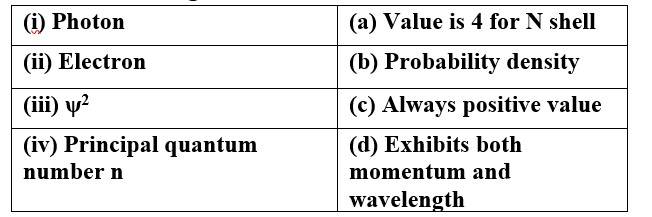
(i) Photon (d) Exhibits both momentum and wavelength
(ii) Electron (d) Exhibits both momentum and wavelength
(iii) ψ 2 (b) Probability density
(c) Always positive value
(iv) Principal quantum (a) Value is 4 for N shell
number n (c) Always positive value
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
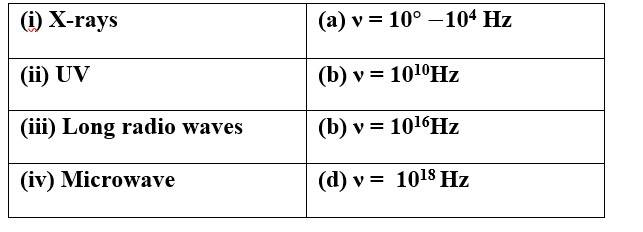
(i) X-rays (d) ν = 1018Hz
(ii) UV (c) ν = 1016Hz
(iii) Long radio waves (a) ν = 10 -104 Hz
(iv)Microwave (b) ν = 1010 Hz
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
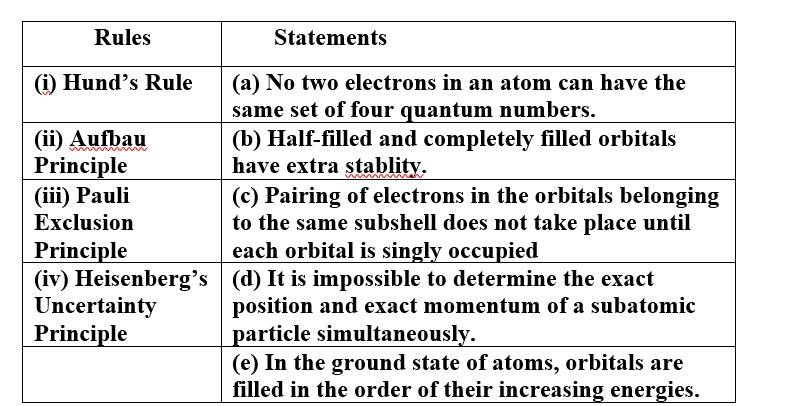
(i) Hund's Rule (c) Pairing of electrons in the orbitals belonging to the same subshell does not take place until each
orbital is singly occupied.
(ii) Aufbau Principle (e) In the ground state of atoms, orbitals are filled in the order of their increasing energies.
(iii)
New answer posted
7 months agoContributor-Level 10
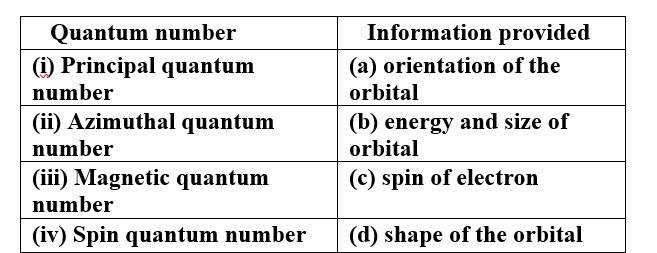
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Principal quantum number (b) energy and size of orbital
(ii) Azimuthal quantum number (d) shape of the orbital
(iii) Magnetic quantum number (a) orientation of the orbital
(iv) Spin quantum number (c) spin of electron
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers