The S-block Elements
Get insights from 196 questions on The S-block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The S-block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Lithium salts are extensively hydrated due to high hydration enthalpy of Li+
(order of polarizing power)
New answer posted
6 months agoContributor-Level 10
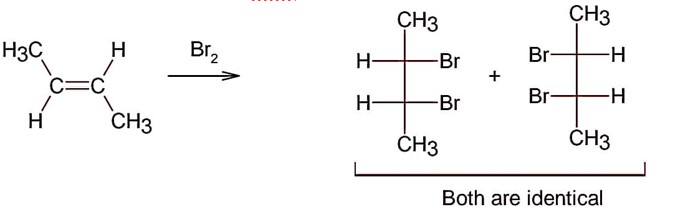
Electrophilic addition of bromine to an alkene is anti-addition, in which cis-alkene gives two enantiomers and trans – alkene gives meso form
Here; trans-but-2-ene will give meso products
New answer posted
6 months agoContributor-Level 10
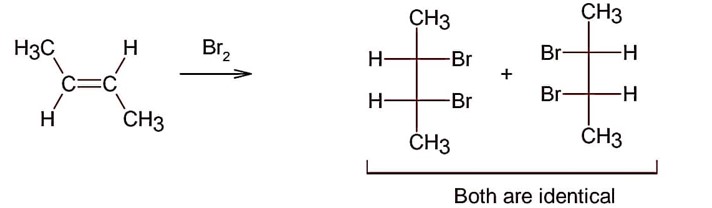
Electrophilic addition of bromine to an alkene is anti-addition, in which cis-alkene gives two enantiomers and trans – alkene gives meso form
Here; trans-but-2-ene will give meso products
New answer posted
6 months agoContributor-Level 10
w = 20 g
Mole of Na2O=
1 mole of Na2O gives 2 mole of NaOH
mole of Na2O gives moles of NaOH
Molarity of NaOH solution
= 1.29 M
=
Ans. = 13
New answer posted
6 months agoContributor-Level 9
Portland cement contains
Dicalcium silicate = 26%
Tricalcium silicate = 51%
Tricalcium aluminate = 11%
Hence major percentage is of tricalcium silicate
New answer posted
6 months agoContributor-Level 10
(1) Standard enthalpy of formation for alkali metal bromides becomes more negative on descending down the group.
(2) Standard enthalpy of formation for LiF is most negative among alkali metal fluorides.
(3) In case of Csl, lattice energy is less but Cs+ having less hydration energy due to which it is less soluble in water.
(4) For alkali metal fluorides, the solubility in water increases from Li to Cs. LiF is least soluble in water.
New answer posted
6 months agoContributor-Level 10
Number of water molecule
Gypsum :- CaSO4.2H2O => 2
Plaster of paris :- CaSO4.
Dead burnt plaster : CaSO4 => 0
New answer posted
6 months agoContributor-Level 10
De-ionised water means dissolved minerals free water which is prepared by synthetic resin method. Where cation exchanged by H+ and anion exchanged by OH- of resin.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers