Thermal Properties of Matter
Get insights from 55 questions on Thermal Properties of Matter, answered by students, alumni, and experts. You may also ask and answer any question you like about Thermal Properties of Matter
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Decrease in temperature = 57-37= 200C
Coefficient of linear expansion = 1.7 oC
Bulk modulus for copper B = 140
Coefficient of cubical expansion = 3 = 5.1
Let initial volume of the cavity be V and its volume increases by due to increase in temperature.
Thermal stress produced = B
= B
= 140
= 1428 2
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
As difference in volume is constant
By considering the diagram
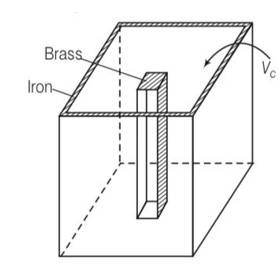
Let Vio , Vbo be the volume of iron and brass vessel at 00C
Vi,Vb be the volume of iron and brass vessel at 0C
be the coefficient of volume expansion of iron and brass.
Vio -Vbo= 100cc= Vi-Vb
Vi =Vio(1+ i )
Vb =Vbo(1+ b )
Vi-Vb = (Vio -Vbo)+
Since Vi-Vb= constant
Vio i= Vbo
Using above equations
Vbo = 144.9cc
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
As liron-lbrass =10cm= constant at all temperature
Let lo be the length of temperature at 00C and l be the length after change in temperature
liron-lbrass =10cm
liron (1+ )-lbrass (1+ )=10cm
Iiron iron= Ibrass brass
=1.8/1.2=3/2
Lbrass=20cm and liron=30cm
New answer posted
8 months agoContributor-Level 10
11.22
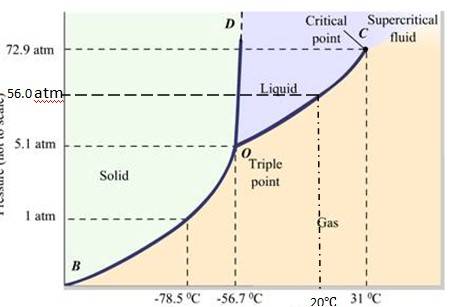
The fusion and boiling points are given by the intersection point where this parallel line cuts the fusion and vaporization curves. It departs from ideal gas behavior as pressure increases.
(a) At 1 atm pressure and at – 60 °C, it lies left of -56.6 (triple point O). Hence, it lies in the region of vapour and solid phases. Thus, CO2 condenses into solid state directly, without going through liquid phases.
(b) At 4 atm pressure, CO2 lies below 5.11 atm (triple point O). Hence it lies in the region of vaporous and solid phases. Thus, CO2 condenses into solid state directly, without going through liquid state.
New answer posted
8 months agoContributor-Level 10
11.21
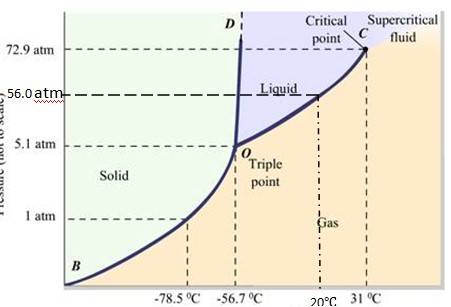
(a) The P-T phase diagram for CO2 is shown here. O is the triple point of the CO2 phase diagram. This means that at the temperature and pressure corresponding to this point, the solid, liquid and vaporous phases of CO2 exists in equilibrium.
(b) The fusion and the boiling points of CO2 decreases with a decrease in pressure.
(c) The critical temperature and critical pressure of CO2 are 31 72.9 atm respectively. Even if it is compressed to a pressure greater than 72.9 atm, CO2 will not liquefy above the critical temperature.
(d) It can be concluded from the P-T phase diagram of CO2 that:
CO2 is gaseous at -70 , under 1 atm pr
New answer posted
8 months agoContributor-Level 10
11.20 According to Newton’s law of cooling, we have
= K(T -
Temperature of the surroundings =
K is a constant
The temperature of the body falls from 80
Integrating equation (i), we get
The temperature of the body falls from 60
Hence, we get:
Equating equations (ii) and (iii), we get,
Hence, the time taken to cool the body from 60
New answer posted
8 months agoContributor-Level 10
11.19 (a) A body with a large reflectivity is a bad absorber. A bad absorber will in turn be a poor emitter of radiations.
(b) Brass is a good conductor of heat, when one touches a brass tumbler, heat is conducted from the body to the brass tumbler easily. Hence, the temperature of the body reduces to a colder value and one feels cold.
On the other hand, wood is a poor conductor of heat. Very little heat is conducted from the body to the wooden tray. Resulting in negligible drop in body temperature.
Thus a brass tumbler feels colder than a wooden tray on a chilly day.
(c) Black body radiation equation is given by:
E =
New answer posted
8 months agoContributor-Level 10
11.18 Base area of the boiler, A = 0.15 m2
Thickness of the boiler, l = 1.0 cm = 0.01 m
Boiling rate of water, R = 6.0 kg/min
Let us assume the mass of the boiling water, m = 6 kg and the time to boil, t = 1 min = 60 s
Thermal conductivity of brass, K = 109 J s–1 m–1 K–1
The amount of heat flowing into water through the brass base of the boiler is given by:
Heat required for boiling water
By equating for
6
New answer posted
8 months agoContributor-Level 10
11.17 Size of the sides of cubical ice box, s = 30 cm =0.3 m
Thickness of the icebox, l = 5 cm = 0.05 m
Mass of ice kept in the box, m = 4 kg
Time gap, t = 6 h = 6
Outside temperature, T = 45 °C
Coefficient of thermal conductivity of thermocole, K = 0.01 J
Heat of fusion of water, L = 335
Let m' be the mass of the ice melts in 6 h
The amount of heat lost by the food:
A = Surface area of the box = 6
We also know
Hence the amount of ice remains after 6 h = 4
New answer posted
8 months agoContributor-Level 10
11.16 Initial body temp of the child,
Final body temp of the child,
Change in temperature,
Time taken t achieve this temperature, t = 20 min
Specific heat of human body = Specific heat of water, c = 1000 cal/kg/
Latent heat of evaporation of water, L = 580 cal/g
Mass of the child, m = 30 kg
The heat lost by the child is given as
Let
Loss of heat
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
