Aldehydes, Ketones and Carboxylic Acids
Get insights from 93 questions on Aldehydes, Ketones and Carboxylic Acids, answered by students, alumni, and experts. You may also ask and answer any question you like about Aldehydes, Ketones and Carboxylic Acids
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: A and B
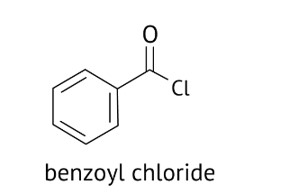
A: Benzophenone can be obtained by Friedel-craft acylation reaction
B: Benzophenone can also be obtained by the reaction between benzoyl chloride and diphenyl cadmium.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: A and C
Grignard Reaction is the addition of an organomagnesium halide to form a tertiary or secondary alcohol.
Canizzaro's Reaction involves the base induced disproportionation of two molecules. Aldol Condensation is an organic reaction when an enolate ion reacts with a carbonyl compound to form beta hydroxy ketone with dehydration to give a conjugated enone.
The HVZ reaction involves alpha bromination of carboxylic acids and this reacts with chlorine or bromine in presence of a small amount of red phosphorus to give alpha halo carboxylic acids.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Solution: (b, d)
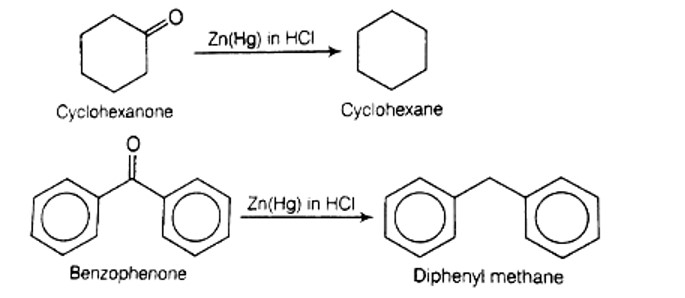
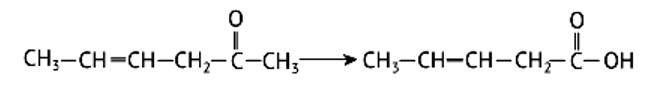
Clemmensen reduction is used to convert cyclohexanone into cyclohexane and benzophenone into diphenyl methane .
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: B and C
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: B and D
Consider the following product CH3 -CHO C and O are broken into positive and negative bonds. C should have one condition: hydrogen must be available; if it isn't, aldol condensation will not occur. When two products are mixed with the availability of - hydrogen, the positive and negative bonds between carbon and hydrogen are exchanged, and water (H20) is eliminated, resulting in aldol condensation.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: A
The Clemmensen Reduction method is used to reduce a carbonyl group to CH2. Amalgams are a type of reducing agent that has been around for a long time. In the Clemmensen reduction, hydrogen chloride acts as an acid, protonating the ketone and transferring the electrons from the zinc amalgam to the carbon atoms
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: B
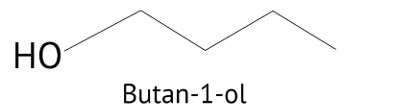
When a primary alcohol, such as Butan-1-ol, reacts with a powerful oxidising agent, such as KMnO4, it produces carboxylic acid, however when a secondary alcohol, such as Butan-2-ol, is oxidised under the pressure of KMnO4, it produces ketone. As a result, option B is right.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: C
I2 and NaOH It is an iodoform reaction involving methyl ketones, the solution is the correct reagent. Because the reactants and product have double bonds, double bonds do not work throughout the reaction. There are also ketone and carboxylic groups present. The ketonic group is oxidised under the carboxylic group during the process.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: B
The position of a functional group in the same carbon chain differs in positional isomerism. The functional group in the chemical or the unsaturated double or triple bond in the compound can help us figure it out.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option: D
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers