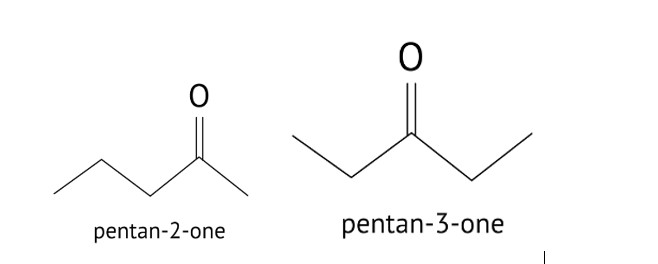
Aldehydes, Ketones and Carboxylic Acids
Get insights from 93 questions on Aldehydes, Ketones and Carboxylic Acids, answered by students, alumni, and experts. You may also ask and answer any question you like about Aldehydes, Ketones and Carboxylic Acids
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
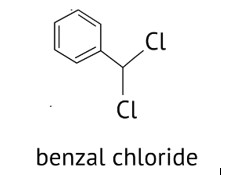
Benzal chloride can be made by chlorinating toluene in the presence of sunshine and then using the hydrolysis process to obtain benzaldehyde. This method can be used to make benzaldehyde in a commercial setting.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans:
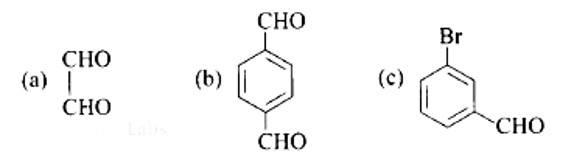
A: Ethane-1, 2-dial
B: Benzene-1, 4-carbaldehyde
C: 3-Bromobenzaldehyde
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
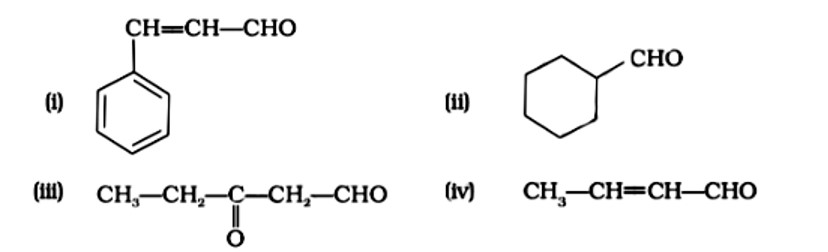
(i) 3-Phenylprop-2-ene-1-al.
(ii) Cyclohexanecarbaldehyde
(iii) 3-Oxopentan-1-al
(iv) IUPAC name: But-2-enal
New answer posted
6 months agoContributor-Level 10
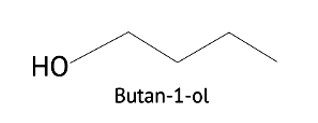
Butanol is a four-carbon aldehyde, with the aldehyde group on the fourth carbon. It has a functional group and a carbonyl group that interacts dipole-dipole. Because butanol possesses a polar O-H bond, it exhibits intermolecular H bonding, which is not feasible in butanal due to the lack of a polar bond. The boiling point of butanol is higher than that of butan-1-ol.
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: Liquid 'A' reduces ammoniacal silver nitrate and 'B' is a ketone which forms white crystalline solid on treatment with hydrogen sulphite
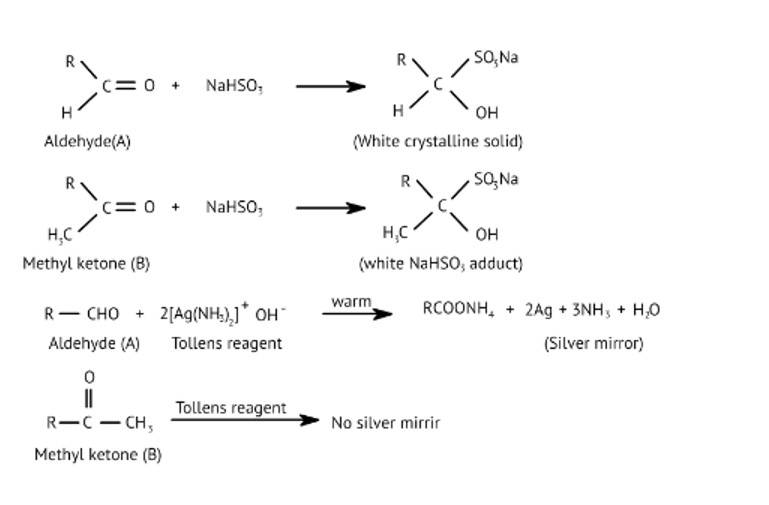
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: Functional isomers

Compound I will react faster with HCN due to less steric hindrance and greater positive charge on carbon atom of carbonyl group. Two methyl groups increase electron density on carbonyl carbon in compounds II hence the rate of nucleophilic attack is less.
Mechanism of the reaction:
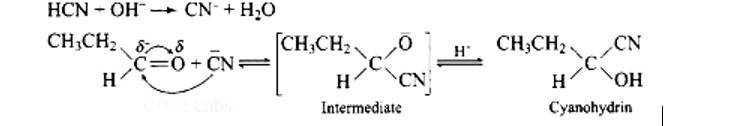
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
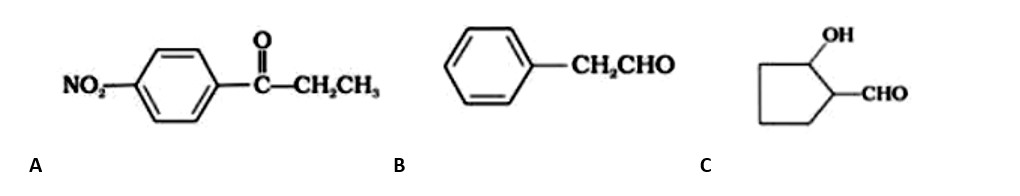
Ans: The aromatic compound 'A' does not give Tollen's reagent test, it is not an aromatic aldehyde. As it responds to an iodoform test called methyl ketone.
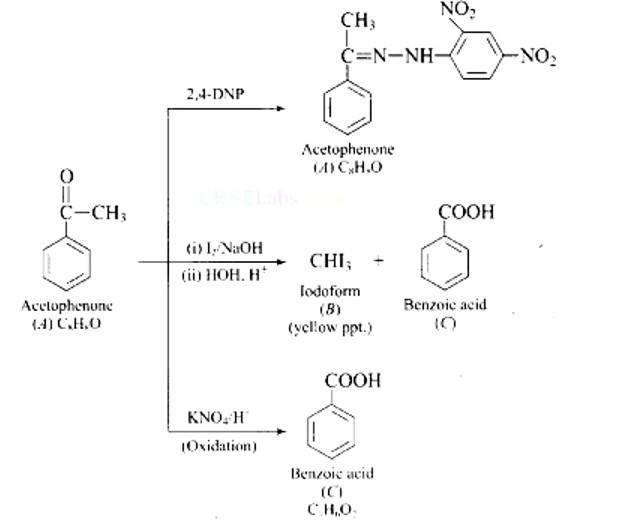
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans:
Fehling's test is positive when compound 'B' is used. It gives an iodoform test and proves that it is an aldehyde. Because it fails Fehling's test, compound 'C' is a ketone.
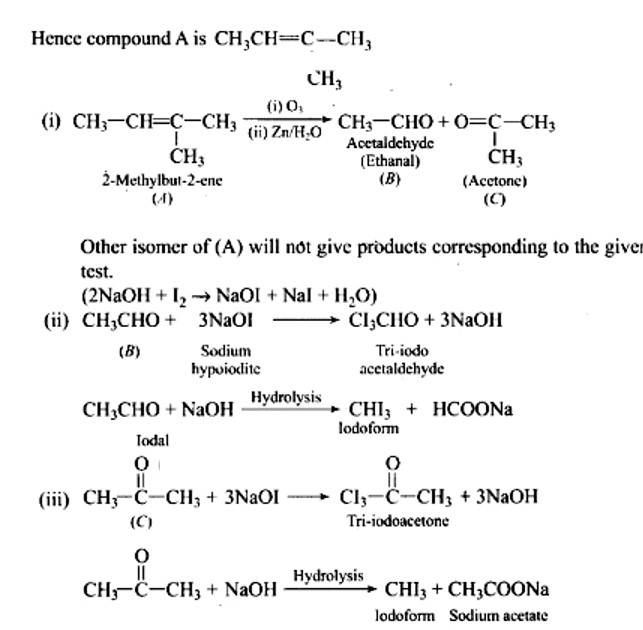
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers