Aldehydes, Ketones and Carboxylic Acids
Get insights from 93 questions on Aldehydes, Ketones and Carboxylic Acids, answered by students, alumni, and experts. You may also ask and answer any question you like about Aldehydes, Ketones and Carboxylic Acids
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
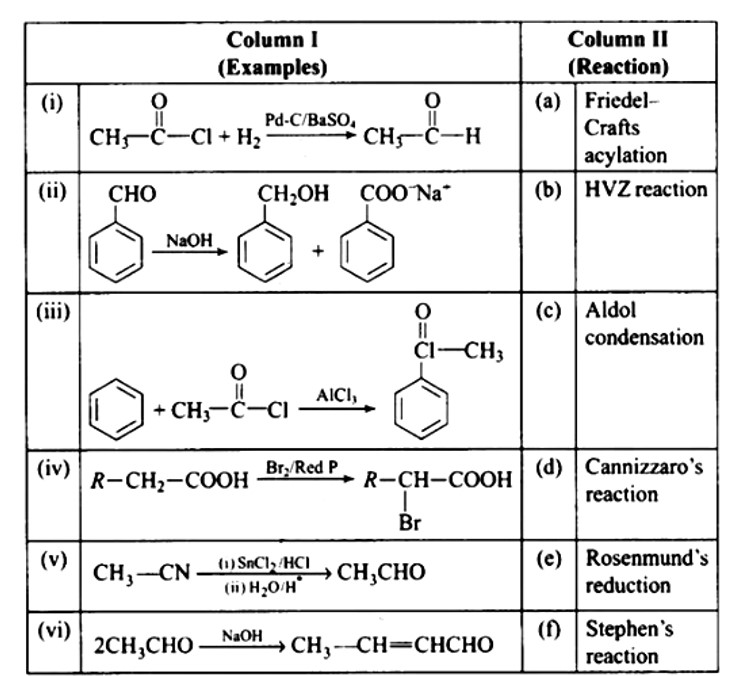
Ans: (i) — (e); (ii) — (d); (iii) — (a); (iv) — (b); (v) — (f); (vi) — (c)
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
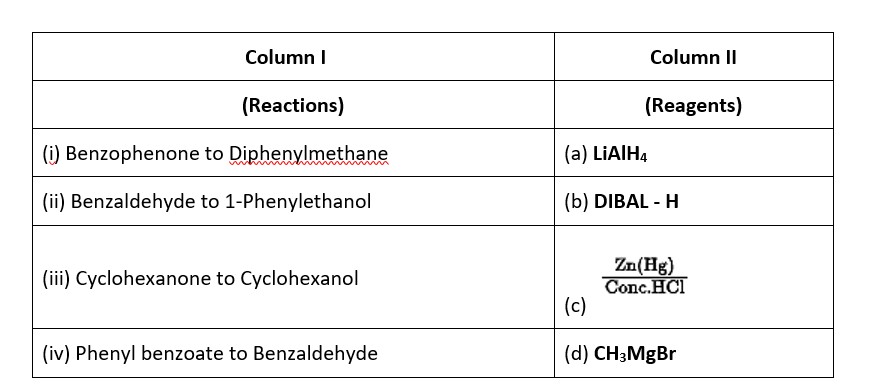
Ans: (i) → (c); (ii) → (d); (iii) → (a); (iv) → (b).
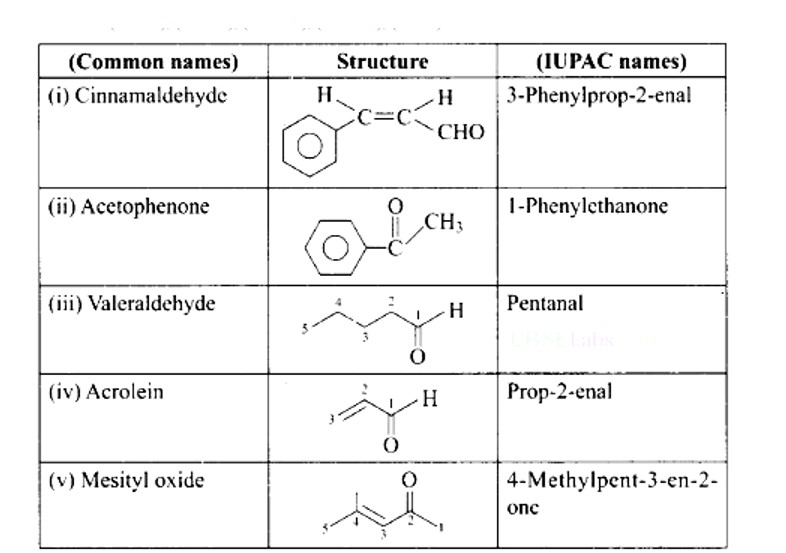
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
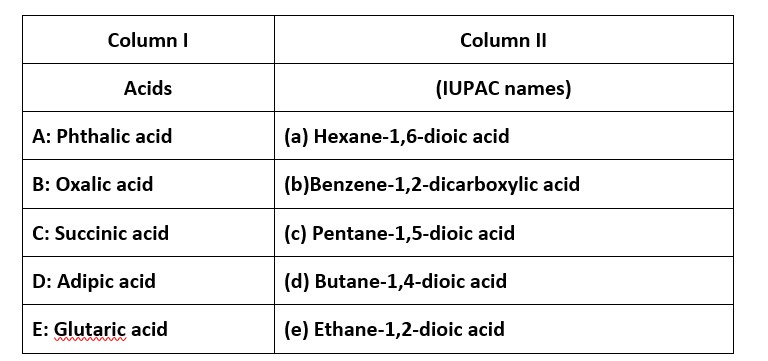
Ans: (i) → (b); (ii) → (e); (iii) → (d); (iv) → (a); (v) → (c)
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
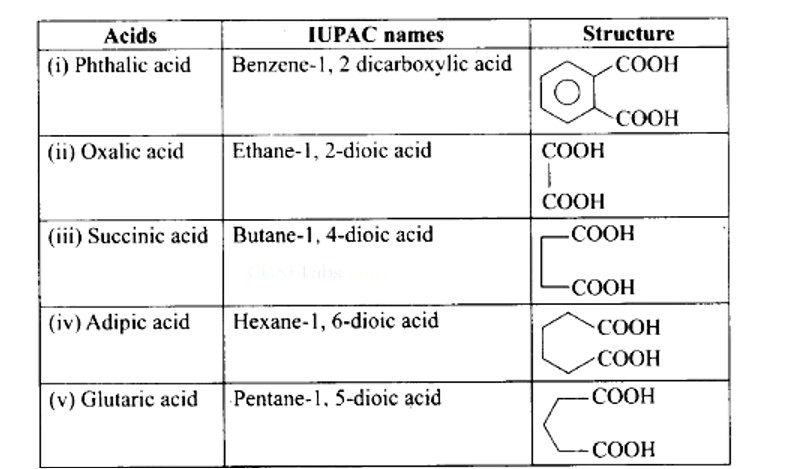
Ans: (i) → (d); (ii) → (e); (iii) → (a); (iv) → (b); (v) → (c)
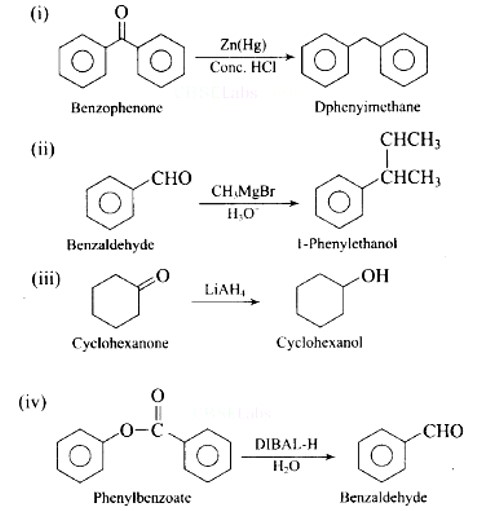
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: D
The silver mirror test can be used to determine Tollen's. [Ag (NH3)2] + OH - . Only aldehydes, not ketones, react with Tollen's reagent to create silver.
A silver mirror test is not given with this affirmative test.
The carbonyl group is present in both aldehyde and ketone.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: C
There is no alpha hydrogen atom in the Cannizaro process. Formaldehyde and aromatic aldehydes do not contain alpha hydrogen. It proceeds through the Cannizaro reaction. Formaldehyde is the most reactive of all aldehydes.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: D
Because of the presence of electron withdrawing carbonyl groups, the alpha hydrogen atom in carbonyl compounds is acidic. In nature, hydrogen is very acidic.
Because the cation is released in the form of H+ , the anion created after the loss of the -hydrogen atom is resonance stabilised.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: E
The two reactions produce different products for different reasons. One involves the oxidation of aldehydes, while the other involves the reduction of carboxylic acids. Aldehydes are easily oxidised by mild oxidising agents, hence compounds containing - CHO are rapidly oxidised.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: A
The smallest aldehyde is formaldehyde. It features a triangular planar shape with two bond pairs and no lone pairings. C and O share one pair of electrons in their double bond. As a result, the C atom is sp2 hybridised. As a result, both Assertion and Reason are true.
New answer posted
6 months agoContributor-Level 10
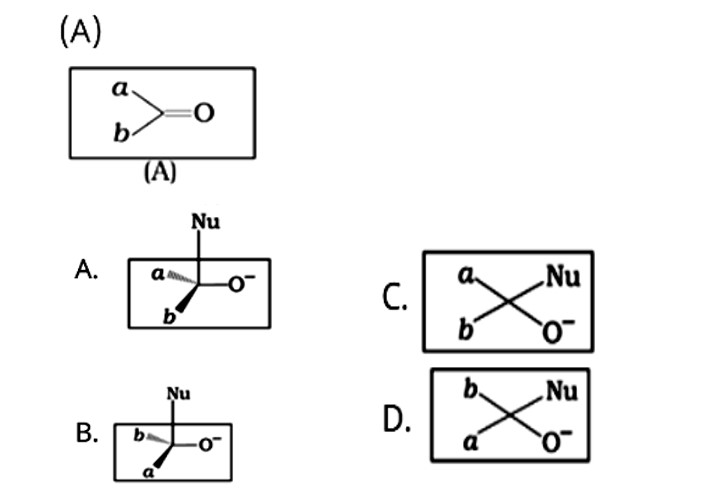
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: A and B
The given compound is a planar molecule with sp2 hybridised carbon. Carbon atoms are attacked by nucleophiles. If the nucleophile approaches from the front, the A and B molecules shift to the front and back positions, respectively, and the carbon becomes tetrahedral. Another alternative is that A and B molecules will be located above and below the plane, respectively. As a result, the choices C and D aren't represented as planar molecules.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers