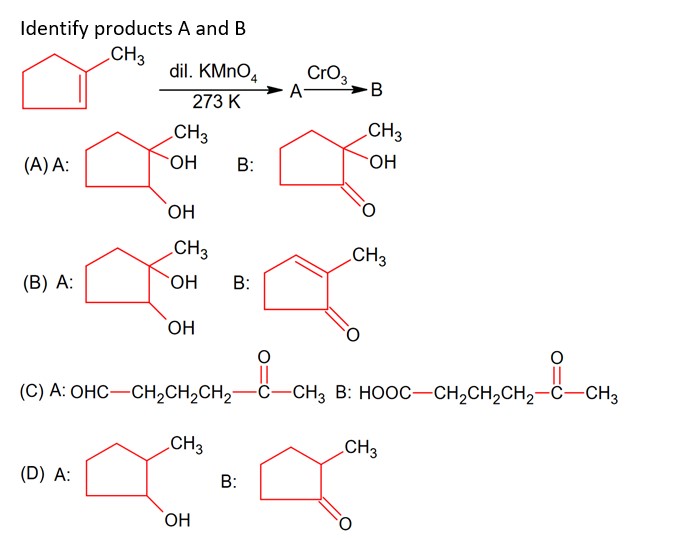
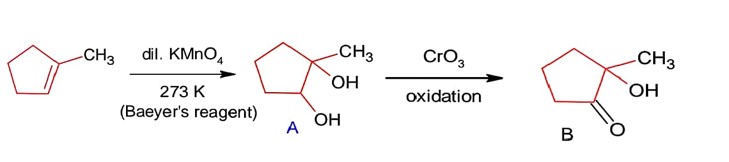
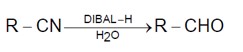
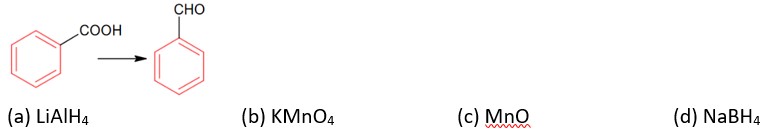Aldehydes, Ketones and Carboxylic Acids
Get insights from 184 questions on Aldehydes, Ketones and Carboxylic Acids, answered by students, alumni, and experts. You may also ask and answer any question you like about Aldehydes, Ketones and Carboxylic Acids
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
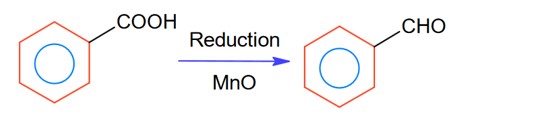
Tollen's reagent oxidises aldehydes into carboxylate ion whereas ketone is not oxidised by tollen's reagent.
New answer posted
4 months agoContributor-Level 10
CH? – CH? – COO? Na? - (NaOH, CaO, Heat)-> CH? – CH? + Na? CO?
Decarboxylation takes place by soda-lime (NaOH + CaO)
New answer posted
4 months agoContributor-Level 10
Teflon are prepared by addition polymerization from tetrafluroethene.
CF? = CF? - (catalyst, High pressure)-> [–CF? – CF? –]? Teflon
Nylon-66, Novolac, Dacron are prepared by condensation polymerisation.
New answer posted
4 months agoContributor-Level 10
π = iCRT
P? = 1 * (10/180) * R * T (For Glucose)
P? = 1 * (10/60) * R * T (For Urea)
P? = 1 * (10/342) * R * T (For Sucrose)
∴ P? > P? > P?
New answer posted
4 months agoContributor-Level 10
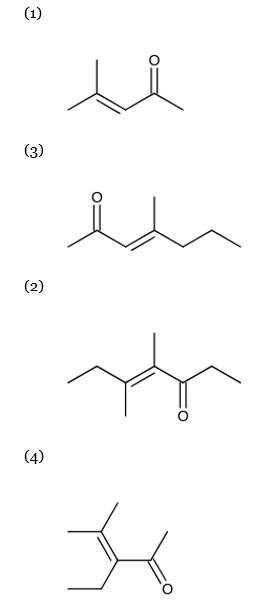
When acetone & 2-pentanone are added in a base for an aldol condensation reaction there are several possibilities that may arise for product formation.
But structure in choice (2) is not possible. It will be possible with 3-pentanone.
New answer posted
4 months agoContributor-Level 10
As compared to hydrocarbons of similar mass aldehydes and ketones will have greater dipole-dipole interactions.
New answer posted
4 months agoContributor-Level 9
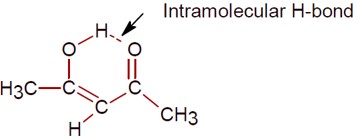
The enol form of acetone exists in less than 0.1% quantity, since its keto form is highly stable. But in the case of acetylacetone, the enol form is stabilized by intramolecular H-bonding, so its quantity increases to approximately 15%.
The intramolecular H-bond in the enol form of acetylacetone is shown.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers