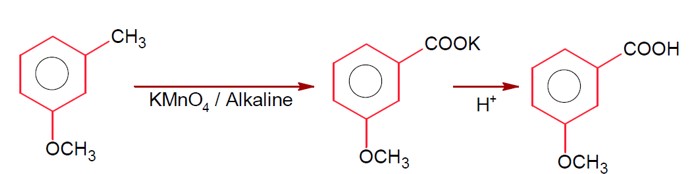
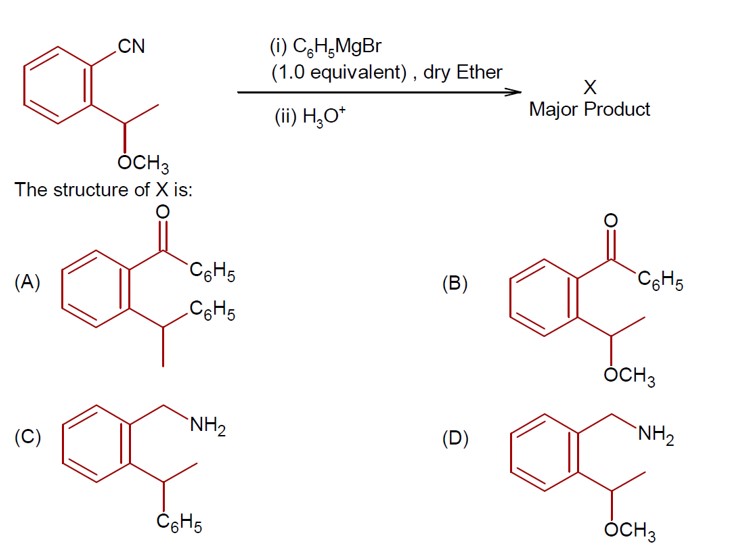
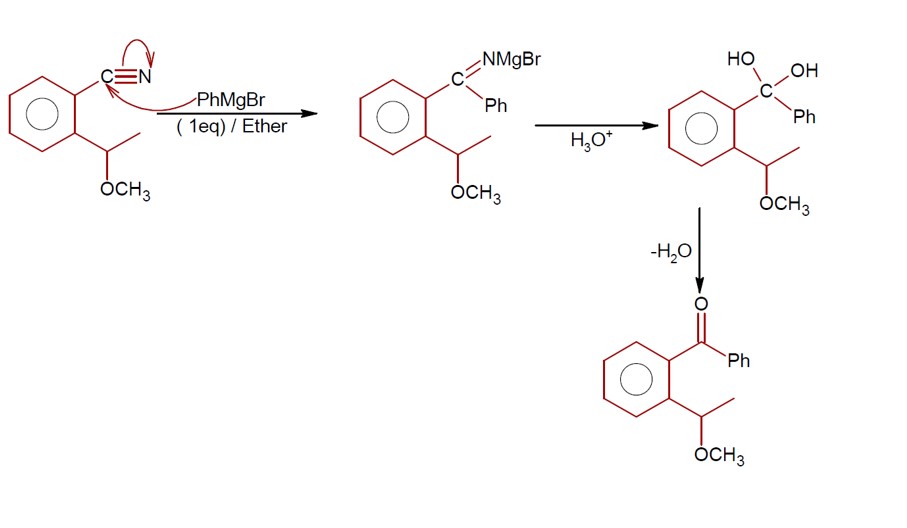
Aldehydes, Ketones and Carboxylic Acids
Get insights from 184 questions on Aldehydes, Ketones and Carboxylic Acids, answered by students, alumni, and experts. You may also ask and answer any question you like about Aldehydes, Ketones and Carboxylic Acids
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 9
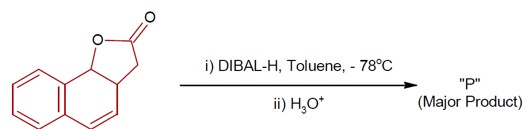
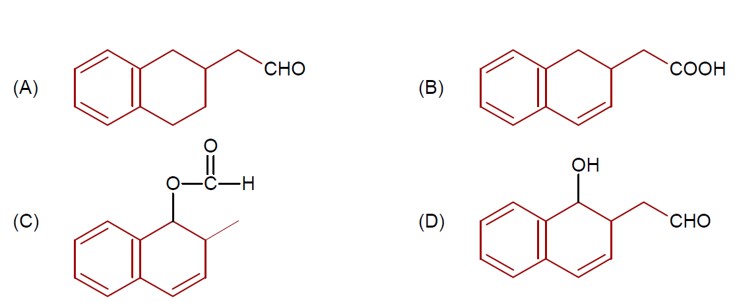
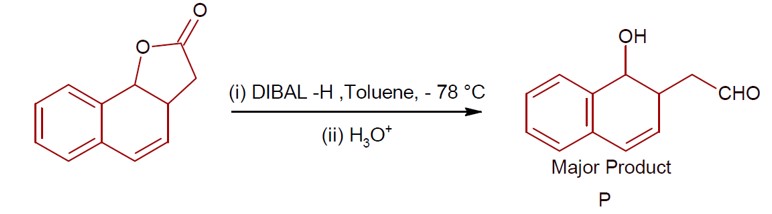
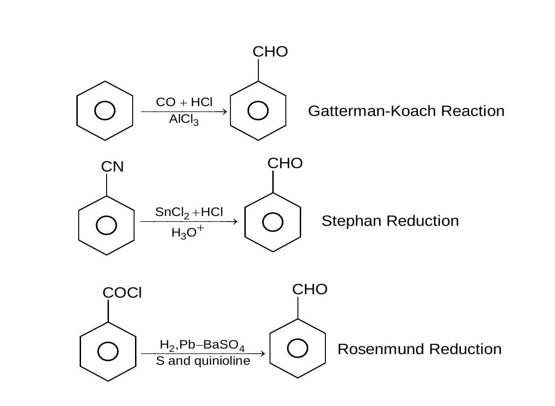
DIBAL-H at low temperature in a non-polar solvent, followed by hydrolysis, reduces esters to an aldehyde and an alcohol as a byproduct. The reaction shown is:
New answer posted
4 months agoContributor-Level 9
In Tollen's test for aldehyde, aldehyde is oxidized to carboxylic acid salt as:
R – CHO + H? O →R – COO? + 3H? + 2e?
So; 2e? are transferred per aldhyde group.
New answer posted
4 months agoContributor-Level 9
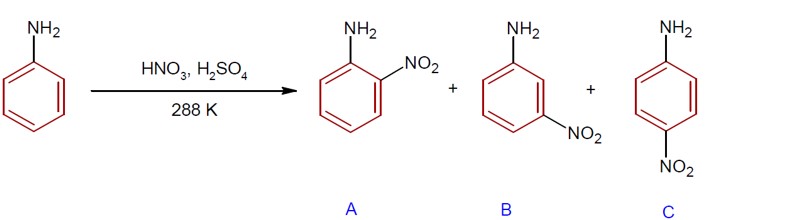
During nitration of aniline, meta- nitroaniline is also formed as product due to formation of –NH? group. The percentage of p, m and o product is 51%, 47% and 2% respectively
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 9
o Chlorophyll: Magnesium present in chlorophyll.
o Vitamin B? : Cobalt (cyanocobalamin).
o Anticancer drug: Platinum (Coordination compound of platinum).
o Grubbs catalyst: Ruthenium.
New answer posted
4 months agoContributor-Level 10
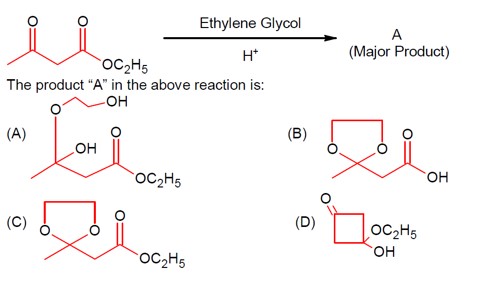
A more reactive carbonyl, i.e., a ketone, is masked with ethylene glycol first in an acidic medium.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers