Biomolecules
Get insights from 151 questions on Biomolecules, answered by students, alumni, and experts. You may also ask and answer any question you like about Biomolecules
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
14.8
A DNA is a double-stranded molecule (two polynucleotide chains whose nitrogenous bases are connected by hydrogen bonds).
In this molecule pairing or connection of bases occurs. Adenine always pairs with thymine, while cytosine always pairs with guanine.
So when DNA is hydrolyzed the amount of adenine produced is exactly equal to the amount of thymine produced, similarly, the amount of cytosine produced is equal to that of guanine.
But as given when RNA is hydrolyzed, there is no relationship between the quantities of different bases obtained. This suggests that RNA is a single-stranded molecule.
New answer posted
8 months agoContributor-Level 10
14.7
When a nucleotide from DNA containing thymine is hydrolyzed β-D-2 deoxyribose and phosphoric acid are obtained.
New answer posted
8 months agoContributor-Level 10
14.6
Vitamin C (ascorbic acid due to extensive –H bonding with water due to the presence of –OH group) is a water-soluble vitamin in contrast to vitamin A, D, E and K which are fat soluble. Also, humans cannot synthesize it due to the lack of specific enzyme and it is rapidly absorbed from the intestine.
Because it is water-soluble, it is not stored in our body to a significant amount but is readily excreted in the urine.
New answer posted
8 months agoContributor-Level 10
14.5
Eggs contain protein and proteins are a polymer in amino acids. When an egg is boiled the protein present inside the egg is denatured (the process of loss of biological activity of protein like their ability of –H bonding when subjected to a physical change like a change in temperature, pH) and coagulated (the process of liquid changing to a solid or semi-solid state). Due to this, the water present in the egg is absorbed by the coagulated protein by –H bonding.
New answer posted
8 months agoContributor-Level 10
14.4
Amino acids are organic compounds containing amine (basic) and carboxyl (acidic) functional group with a specific side chain. Both acidic and basic group are present in the same molecule. In, aqueous solution carboxyl group can lose a proton (H+) and amino group can accept a proton (H+) giving rise to the dipolar ion called as zwitter ion. Zwitter ion is shown below:
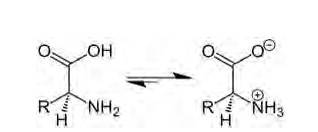
In this zwitter ion there is the presence of both positive as well as negative charge, so there is the development of strong electrostatic force of attraction between the molecules and the water. For this reason solubility of amino acids is higher. Due to strong e
New answer posted
8 months agoContributor-Level 10
14.3
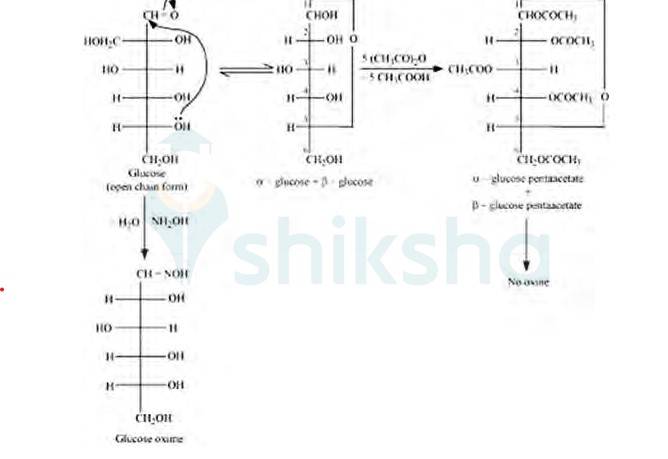
D-glucose reacts with hydroxylamine (NH2OH) to form oxime due to the presence of the aldehyde functional group (-CHO). This is due to the cyclic structure of glucose which forms an open chain structure in an aqueous medium, which then reacts to give an oxime.
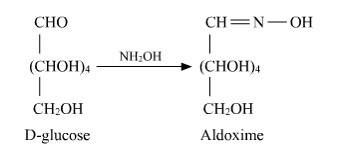
But in case of pentaacetate of D-glucose, it does not form open chain structure in an aqueous medium so it does not react with NH2OH.
New answer posted
8 months agoContributor-Level 10
14.2
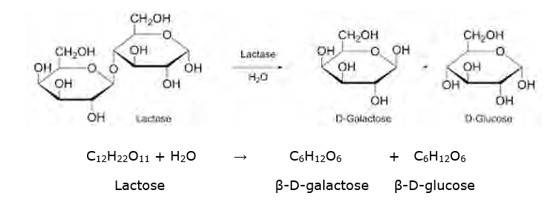
Lactose is a disaccharide carbohydrate (made up of two monosaccharide units) composed of β-D-galactose and β-D-glucose units. Hydrolysis breaks the glycosidic bond converting sucrose into β-D- galactose and β-D-glucose.
NOTE: But however, this reaction is so slow that it takes years for the solution of sucrose to undergo negligible change. Hence an enzyme called sucrase is added to proceed rapidly.
New answer posted
8 months agoContributor-Level 10
14.1
Glucose and sucrose are carbohydrates (optically active polyhydroxy aldehydes or ketones).
Structure of glucose:
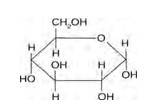
Structure of sucrose:
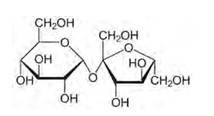
As you can see both the compounds have five –OH and eight –OH groups respectively. These –OH groups are responsible for the extensive hydrogen bonding with water. This –H bonding is responsible for the solubility of glucose and sucrose in water.
In case of cyclohexane or benzene (simple six-membered ring compounds), they do not contain any – OH groups. Hence, they cannot undergo –H bonding with water and are insoluble.
New answer posted
9 months agoBeginner-Level 5
Students ask this question many a times that wehter NCERT are enough or they should follow other reference books. Students should use NCERT books for CBSE Class 12 board exams, NCERT is more than sufficient for mastering the Biomolecules chapter. All key concepts, definitions, and reactions asked in the exams are directly covered in the textbook, WE have provided NCERT Solutions for Class 12 chemistry Biomolecules.
However, for competitive exams like NEET and JEE, while NCERT remains the primary reference, students should also solve additional MCQs from reference books like MTG NCERT at Your Fingertips, NCERT Exemplar, or pra
New answer posted
9 months agoBeginner-Level 5
Chapter 10 Biomolecules of Class 12 Chemistry includes several important topics which are frequently asked in the state and cbses board exams. The most important topics in the Biomolecules chapter include the classification and structure of carbohydrates (monosaccharides, disaccharides, polysaccharides), amino acids and proteins (including peptide bond formation), enzymes and their characteristics, nucleic acids (DNA and RNA structure and components), and vitamins with their types and deficiencies.
Questions often focus on structural identification, naming, and functions of biomolecules, as well as simple reactions like hydrolysis
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
