Chemistry NCERT Exemplar Solutions Class 11th Chapter Three
Get insights from 79 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Three, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Three
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iii)
Gadolinium is a lanthanoid which belongs to the f-block elements with electronic configuration (n-2)f14 (n-1)d0-1ns2
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (i)
As Mg contains fully filled 3s orbital thus its ionisation is higher than that of Na and Al.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (i)
The orbitals nearer to the nucleus possess more shielding effect.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iv)
Actinoids possess Z=90-103
Terbium is lanthanoid.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (ii)
All the given ions are isoelectronic species thus their radii depend upon the charge more the negative charge higher would be the atomic radii and higher the positive charge lesser would be the atomic radii.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The electronegativity of the alkali metals decreases down the group due to the increase of the shells thus Cs (Caesium) is the least electronegative metal in the alkali metals.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The radius of Na+ ion is smaller than that of Na is due to the following reasons:-
(i) The effective nuclear charge of Na+
(ii) The disappearance of 3s orbital from its outermost shell electronic configuration.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Metallic character- Tendency to lose electrons.
Non-Metallic character- Tendency to accept electrons.
Across the period the metallic character decreases whereas non-metallic character increases across the period.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
(a) As the effective nuclear charge increases and shielding effect decreases across the periods thus the electronegativity increases on moving from left to right in the periodic table.
(b) As the number of shells increases down the group thus its ionisation enthalpy decreases down the group.
New answer posted
8 months agoContributor-Level 10
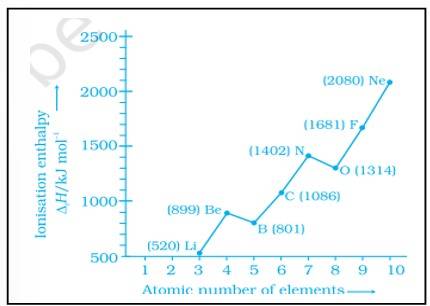
The deviation in the ionization enthalpy of some elements from the general trend can be explained by the points as given below:-
(i) The fully filled and half filled orbital provide extra stability due to the symmetry
(ii) The effective nuclear charge
(iii) The e- - e- repulsion which lead to instability
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers