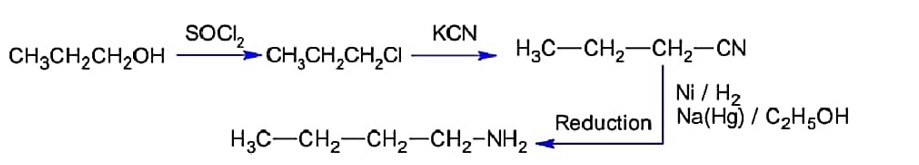
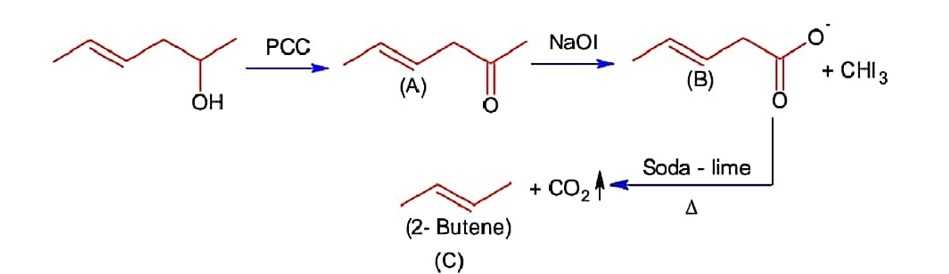
Chemistry NCERT Exemplar Solutions Class 11th Chapter Three
Get insights from 79 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Three, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Three
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
The value of crystal field spilitting energy increases down the group. 5d series member will have more compared to 3d series or 4d series member.
New answer posted
7 months agoContributor-Level 10
Phosphorous can expand its covalency beyond 4, as it has vacant 3d orbitals. Nitrogen can't expand its covelency beyond 4, as it does not posses vacant 3d- orbitals.
New answer posted
7 months agoContributor-Level 10
Fire extinguisher contains sodium Bicarbonate (NaHCO3), also known as baking soda.
New answer posted
7 months agoContributor-Level 10
NaOH is obtained by electrolysis of NaCl. Solution of NaCl using Hg cathode
New answer posted
7 months agoContributor-Level 10
In Hall Heroult process electrolysis is carried out using carbon anode. The liberated
O2 at anode reacts with anode.
Cathode :
Anode :
Net Reaction 2Al2O3 + 3C
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers