Chemistry NCERT Exemplar Solutions Class 11th Chapter Three
Get insights from 79 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Three, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Three
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 9
For the complex K? [Cr (oxalate)? ], the central metal ion is Cr³?
Electronic configuration of Cr (24) is [Ar] 4s¹3d?
Electronic configuration of Cr³? is [Ar] 4s?3d³.
The number of unpaired electrons in Cr³? is 3.
New answer posted
5 months agoContributor-Level 9
Combustion of ethane: C? H? + 7/2 O? → 2CO? + 3H? O.
Moles of C? H? = 3g / 30 g/mol = 0.1 mol.
Moles of H? O produced = 0.3 mol.
Number of H? O molecules = 0.3 * 6.023 * 10²³ ≈ 18.06 * 10²².
So, x = 18.
New answer posted
5 months agoContributor-Level 9
Given K_f = 1.85 K kg mol? ¹ for a solution with molality of 2 m.
ΔT_f = I * K_f * m
3.885 = I * 1.85 * 2
The van't Hoff factor, I = 1.05.
i = 1 + (n-1)α. For an electrolyte dissociating into 2 ions, n=2.
1.05 = 1 + (2-1)α.
The degree of dissociation, α = 0.05 or 50 * 10? ³.
New answer posted
5 months agoContributor-Level 9
3-Hydroxy propanal
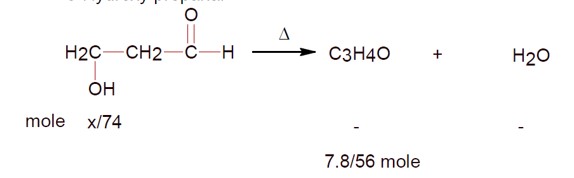
If 7.8g of C? H? O (molar mass 56 g/mol ) is formed, calculate the initial weight of 3-hydroxy propanal (molar mass 74 g/mol ).
Weight = (7.8/56) * 74 * (100/64) [Assuming 64% yield, though the number seems out of place].
Ans ≈ 16 g.
New answer posted
5 months agoContributor-Level 9
AX is a diatomic molecule with a bond order of 2.5.
The compound is NO. The total number of electrons = 15 (7+8).
New answer posted
5 months agoContributor-Level 9
For an acidic buffer solution, pH = pKa + log ( [Base]/ [Acid]).
Given pH = 5.74 and pKa = 4.74.
5.74 = 4.74 + log ( [Base]/1).
1 = log ( [Base]).
[Base] = 10M.
New answer posted
5 months agoContributor-Level 9
For the reaction C? H? → C? H? + H? , calculate the enthalpy change (ΔH).
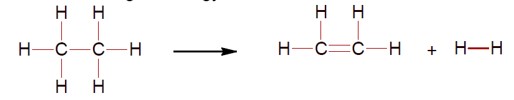
ΔH = [Bond energy (C-C) + 6 * Bond energy (C-H)] - [Bond energy (C=C) + 4 * Bond energy (C-H) + Bond energy (H-H)]
ΔH = 347 + 2 (414) - 611 - 436 = 128 kJ/mol.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers