Chemistry NCERT Exemplar Solutions Class 11th Chapter Two
Get insights from 63 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Two, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Two
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Given, m (mass) = 10g, v (speed) = 90 m/s and accuracy = 4%
Uncertainty in speed = 3.6 ms-1
Uncertainty in position = h/4 πmΔv = 6.626 * 10-34/4 * 3.14 * 10 * 3.6
=1.36 * 10-33m
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans:


New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: We know that
λ= c/υ Given,
υ = 4.620 * 1014 Hz
Thus, λ= c/υ = (3.0 * 108 m/s)/ (4.620*1014 Hz) = 649.4nm
This frequency falls under visible range
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
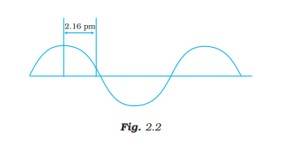
Ans: The wavelength is defined as the distance between two consecutive crests or troughs of a wave, and it is denoted by l .
l = 4*2.16 pm = 8.64 pm
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: We have
λ = h/mv
Thus, the equation signifies that in order to have the same wavelength the electron should have higher velocity as the mass of the proton is higher than that of the electron
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The line spectrum associated with any element possesses lines corresponding to specific wavelengths and these lines are obtained as a result of electronic transitions between the energy levels. Hence, the electrons in these levels have fixed energy i.e., quantized values.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: As per de Broglie, every object in motion has a wave character i.e. every object/matter have dual nature both particle and wave nature. But the wavelengths associated with ordinary objects are so short (because of their large masses) that their wave properties cannot be detected.
Given, m (mass) = 100g, v=100km/hr
We have
λ = h/mv= 238.5 * 10-34 m
As the wavelength associated with the cricket ball is so small that it can't be detected.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: We have V = 109677 [1/ni 2 - 1/nf 2 ] cm-1
Given, ni = 2, nf=4
V =109677 [1/4 - 1/16] = 20564.44 cm-1
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: As per the Hund's rule the half filled and fully filled orbital leads to the extra stability due to the symmetry thus fully filled 3d and half filled 4s is preferred.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans:

We know that energy is inversely proportional to the wavelength

Thus, the increasing order of energy is B
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers