Chemistry NCERT Exemplar Solutions Class 12th Chapter Nine
Get insights from 68 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Nine, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Nine
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
During the electrolysis of dilute H2SO4
In the solid form of dihedral angle is equal to 90.2°.
New answer posted
5 months agoContributor-Level 10
In reducing action, H2O2 changes to O2 because it will oxides but option (A) is in acidic medium, hence answer will be (C).
New answer posted
5 months agoContributor-Level 10
K2 [Cu (CN)4]
Oxidation number of Cu is +1
Cu+ = [Ar]3d10 ® Diamagnetic
New answer posted
5 months agoContributor-Level 10
Tritium is radioactive and it decays into He3 during emission of β-radiation
1T3 → 2He3 + -1e0
New answer posted
6 months agoContributor-Level 10
CN is strong field ligand
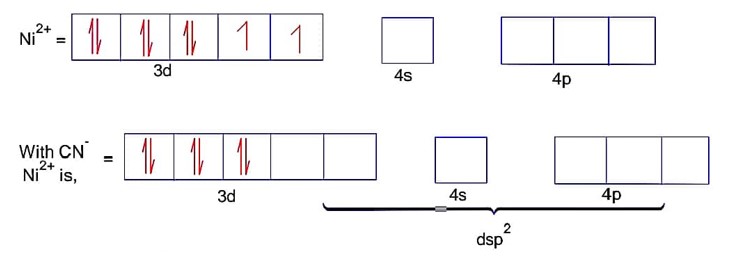
Here; is square planar and diamagnetic.
Ni = 4s23d8Co is strong field ligand.
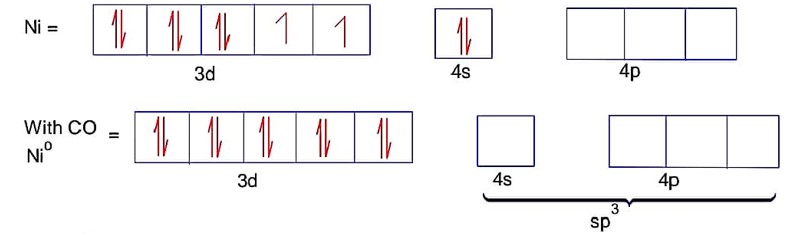
Here ; is tetrahedral and diamagnetic
has 3d8 configuration while has 3d10 configuration.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers