Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Get insights from 127 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
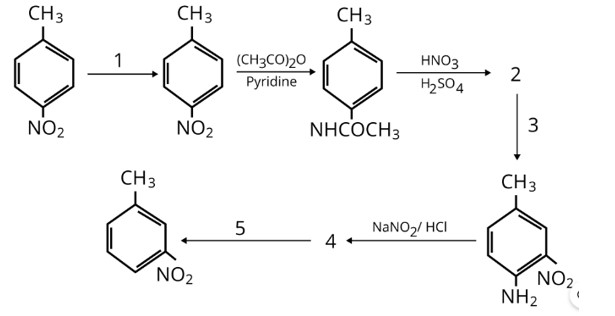
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
6 months agoContributor-Level 10
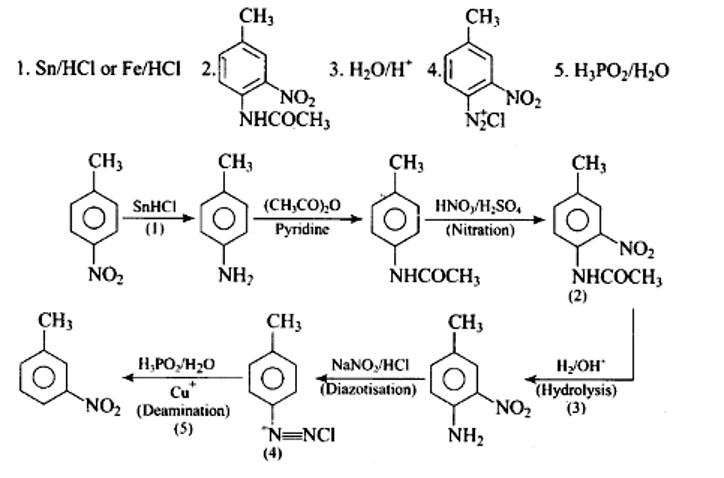
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
6 months agoContributor-Level 10
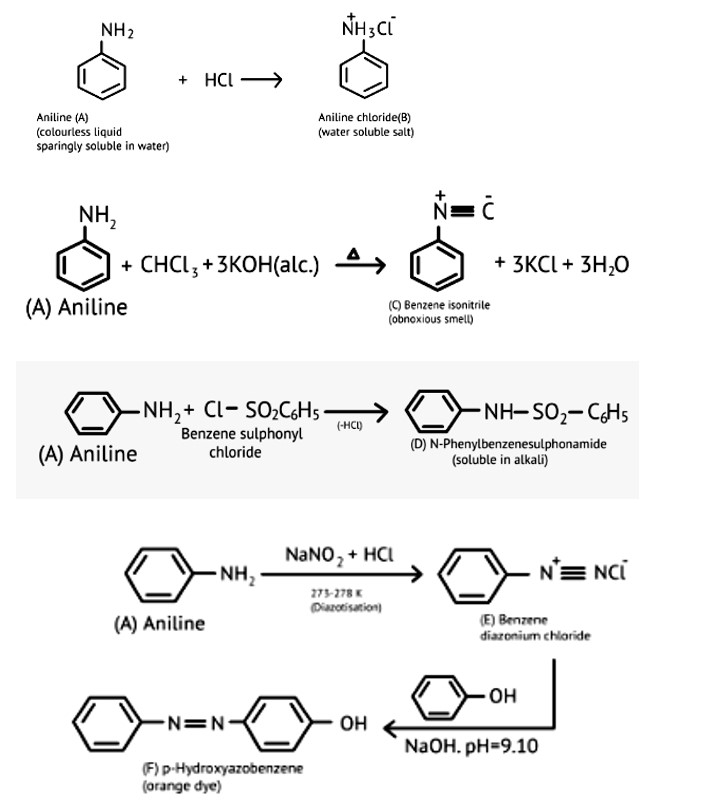
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans:
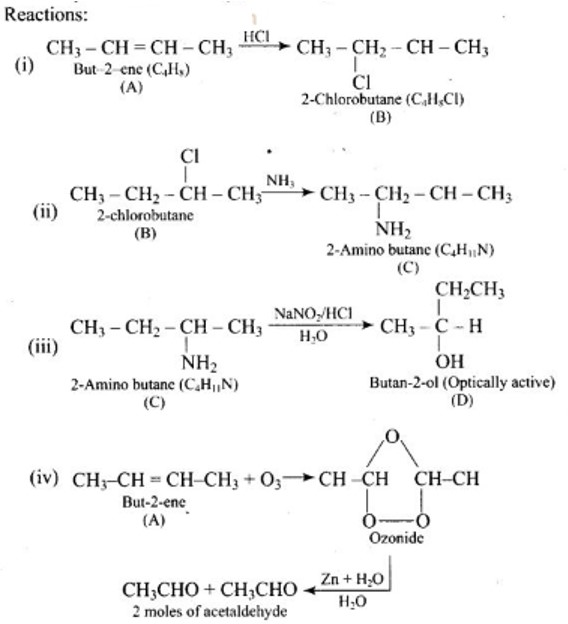
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (d)
Acetanilide is less basic than aniline because electron density of nitrogen is lowered by acetyl group.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (a)
Aromatic primary amines cannot be prepared using Gabriel phthalimide synthesis as aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide. Both the statement's assertion and reason are incorrect, hence the correct answer is a.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (d)
Fe+2HCl→FeCl2+2 [H] FeCl2+H2O (g)→FeO+2HCl
The nascent hydrogen formed act as reducing agent for the reduction of nitro compounds.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (b)
N, N-die ethyl benzene sulphonamide, there is no acidic hydrogen present on the N-atom which can make it soluble. Therefore, it is insoluble in alkali.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (d)
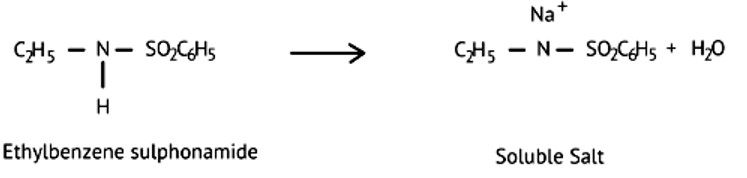
Ethylbenzene sulphonamide is soluble in alkali because it has acidic hydrogen.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (a)
In Hoffmann bromamide degradation reaction as in this reaction primary amide group is treated with halogen first bromine then the halogen substituted amide product is coverts to primary amine with the release of carbon dioxide gas. Both the statements assertion and reason are incorrect therefore the correct option is a.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (c)
In the acryl derivative, the delocalisation of electrons of the nitrogen atom occur over the carbonyl group, this decreases the electron density on the nitrogen atom that it no more perform as nucleophile and don't react with next acylating alkaline molecule.
Therefore, Assertion statement is correct but reason is incorrect.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers