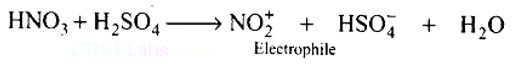Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Get insights from 127 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: Amides are the byproducts of the acylation reaction. The reaction is carried out in the presence of a stronger base than the amine, such as pyridine, which removes the formed HCl and shifts the equilibrium to the right.
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: As the electronegativity of oxygen is more than the electronegativity of a nitrogen atom, the O−H bond is more polar than the N−H bond, therefore MeOH is stronger acid than MeNH2 or MeNH2 is stronger base than MeOH.
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: As −NH2 is a strong activating group, the aniline will readily undergo electrophilic substitution reaction, and it is difficult to cease reaction at the mono substitution stage.
Therefore, the activating group −NH2 is protected by an acetylation process.
The acetylated complex formed utilizes the lone pair of nitrogen and are less available for donation, this helps to carry out the nitration reaction easily.
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
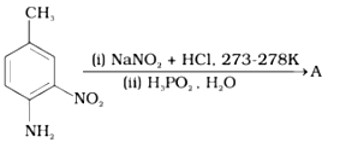
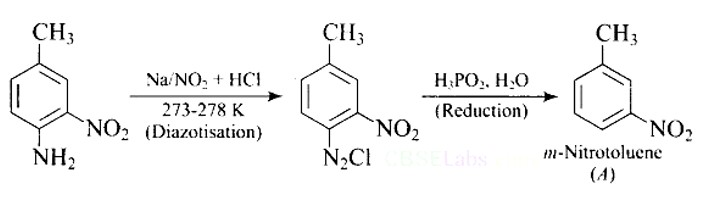
Ans: The reaction of aniline with nitrous acid at 273-278 K produces benzene diazonium chloride. The reaction of sodium nitrite with hydrochloric acid produces nitrous acid in the reaction mixture. Diazotisation is the process of converting primary aromatic amines into diazonium salts. Because of its instability, the diazonium salt is generally not stored or used immediately after preparation.
The crystalline solid benzene diazonium chloride is colorless.
It is easily soluble in water and stable at room temperature, but it reacts with water when warmed.
In the dry state, i
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: Hinsberg's reagent is also known as benzenesulphonyl chloride (C6H5SO2Cl). When it reacts with primary and secondary amines, it produces sulphonamides. Allowing secondary and tertiary amines to react with Hinsberg's reagent allows them to be distinguished (benzenesulphonyl chloride C6H5SO2Cl). Secondary amines react with Hinsberg's reagent to form an alkali-insoluble product. N, N-diethylamine, for example, reacts with Hinsberg's reagent to form N, N-diethylbenzenesulphonamide, which is insoluble in alkalis. Tertiary amines, on the other hand, are unaffected by Hin
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: The best reagents for the conversion of nitrile to primary amine are LiAlH4 and Sodium/Alcohol. By reduction, the nitriles can be converted into a corresponding primary amine.
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
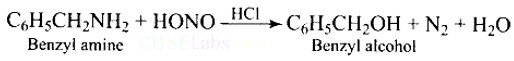
Ans: When Benzyl amine is treated with nitrous acid, firstly BDC is formed which is unstable and decomposes to Benzyl alcohol.
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
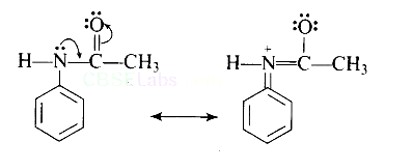
Direct nitration of aniline is not possible on account of oxidation of -NH2 group. However, nitration can be carried after protecting the -NH2 group by acetylation to give acetanilide which is then nitrated and finally hydrolysed to give o- and p-nitroanilines.
The acetyl group being electron withdrawing attracts the lone pair of electrons of the N-atom towards carbonyl group.
As a result, the activating effect -NH2 group is reduced i.e., the lone pair of electrons on nitrogen is less available for donation to benzene ring by resonance. Therefore, acti
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: HNO3 acts as a base in the nitrating mixture (HNO3 + H2SO4) and provides the electrophile.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers