Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Get insights from 127 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (c)
In the acryl derivative, the delocalisation of electrons of the nitrogen atom occur over the carbonyl group, this decreases the electron density on the nitrogen atom that it no more perform as nucleophile and don't react with next acylating alkaline molecule.
Therefore, Assertion statement is correct but reason is incorrect.
New answer posted
6 months agoContributor-Level 10
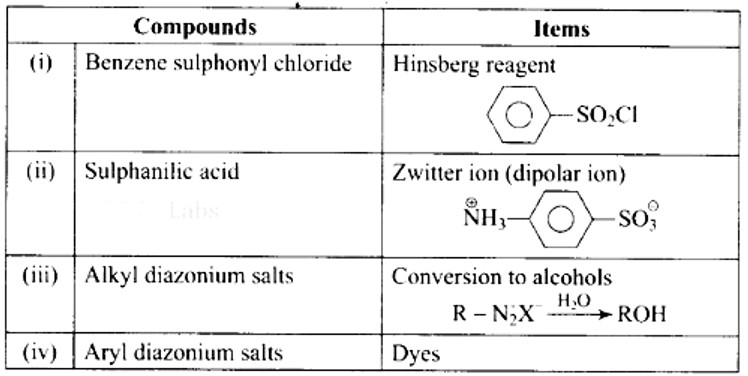
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i)- (b), (ii)- (a), (iii)- (d), (iv)- (c)
New answer posted
6 months agoContributor-Level 10
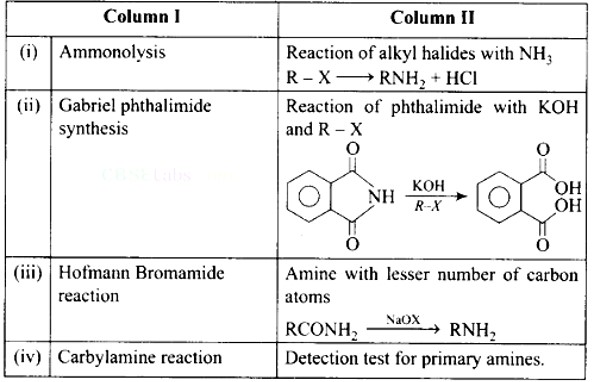
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i)- (d), (ii)- (c), (iii)- (a), (iv)- (b)
New answer posted
6 months agoContributor-Level 10
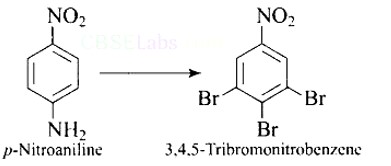
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
6 months agoContributor-Level 10
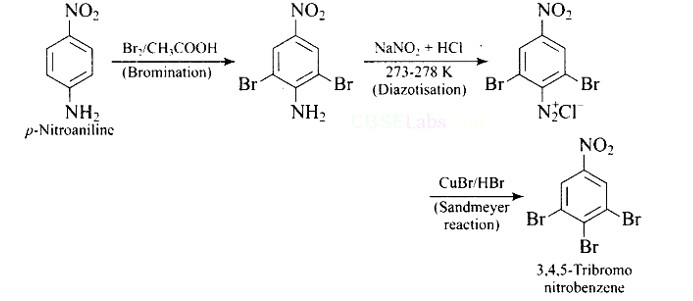
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
6 months agoContributor-Level 10
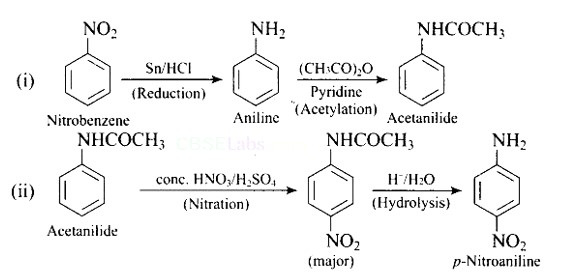
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
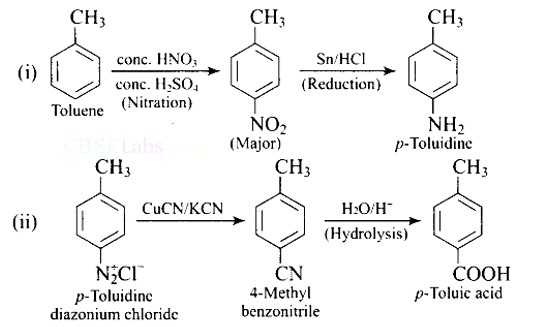
6 months agoContributor-Level 10
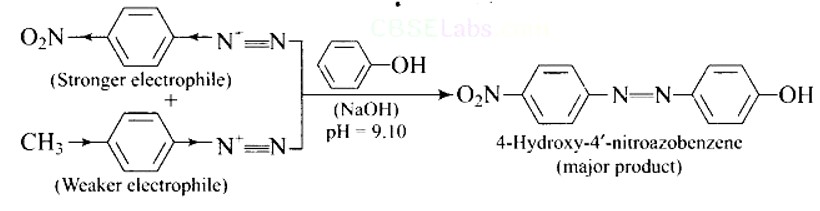
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
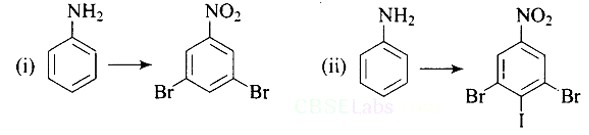
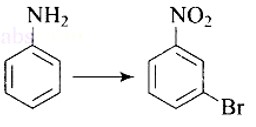
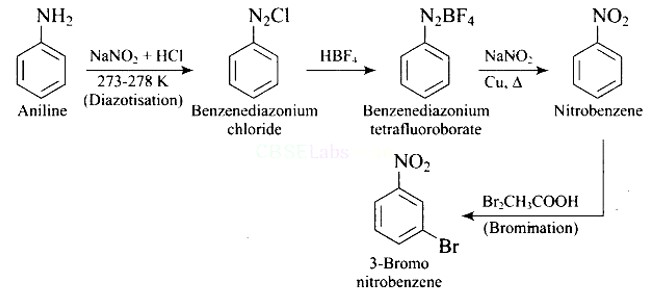
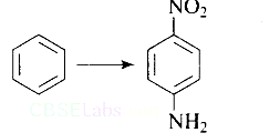
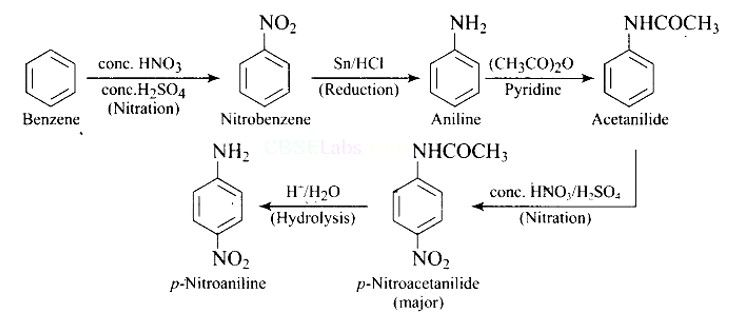
This is an example of an electrophilic aromatic substitution reaction. phenol generates phenoxide ion in alkaline medium, which is more electron-rich than phenol and thus more reactive to electrophilic attack. In this reaction, the electrophile is an aryldiazonium cation.
The faster the reaction, the stronger the electrophile.
The cation p-nitrophenyldiazonium is a stronger electrophile than the cation p-toluene diazonium.
As a result, it preferentially couples with phenol.
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
6 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers