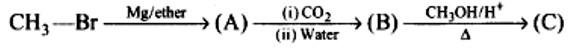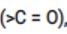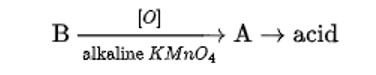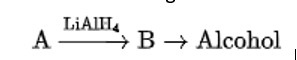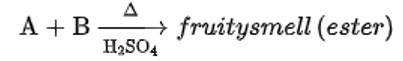Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve
Get insights from 125 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: B
CH3−CH2−C≡CH + H2O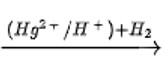
Butan-2-one is produced when butyne is hydrated in the presence of Hg ions as a catalyst. The ketone is the product. After the triple bond is broken, the OH group attaches to the secondary carbocation.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Yes, both reactions look to be the same, but they are not. To convert benzene to benzaldehyde, the Gatterman-Koch reaction is performed. Benzene is treated with an acid chloride in the presence of anhydrous AlCl3 in acylation processes. The Gatterman-Koch reaction is used to make HCOCl in situ by reacting CO with HCl gas in the presence of anhydrous AlCl3.
New answer posted
6 months agoContributor-Level 10
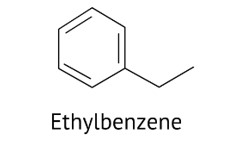
Due to the development of polysubstitution products, ethylbenzene is usually made through acetylation of benzene followed by reduction and direct alkylation.
Due to resonance, the benzene ring must be deactivated. The electrophilic attacking agent is rarely produced by the benzene ring.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
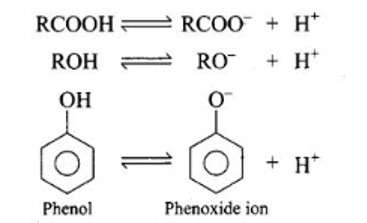
Although all of them have a hydrogen atom connected to an oxygen atom (—O—H), carboxylic acids are more acidic than alcohols or phenols. This can be explained by the conjugate base's stability after H+ ions have been removed from the acid and phenol.
This resonance hybrid may be presented as:-
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
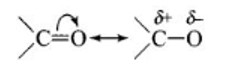
The presence of a lone pair of electrons on an atom of the OH group diminishes the electrophilic character of C through resonance; carboxylic acids contain a carbonyl group. As a result, the carbonyl atom's partial positive charge is lowered.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Solution: Carbonyl group is polar in nature. Due to larger electronegativity of oxygen as compared to carbon, carbon acquires partial positive charge while O acquires partial negative charge.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
NO2CH2COOH > FCH2COOH > C6H5COOH
To begin, determine whether it has an acidic strength associated with it, and whether it is an electron donating or electron withdrawing group. Acidic strength increases as the number of electron withdrawing groups increases. The acidic strength reduces as the electron donating group increases. In decreasing order, the most influential acidic strength is −NO2> −F> −C6H5.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
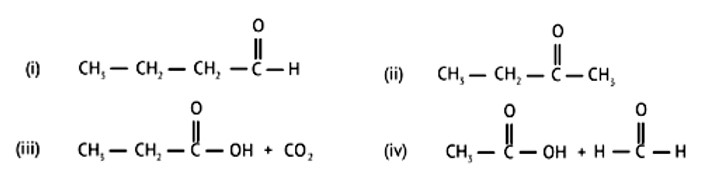
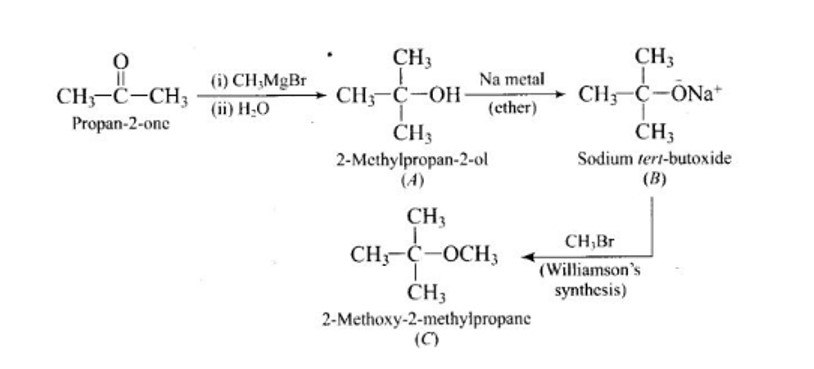
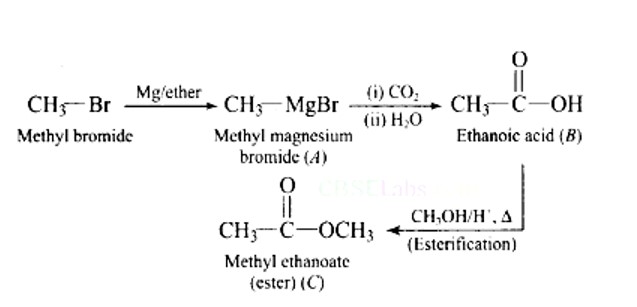
When alkaline KMnO4 oxidises compound "B" with compound "A," acid is formed.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers