Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve
Get insights from 125 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
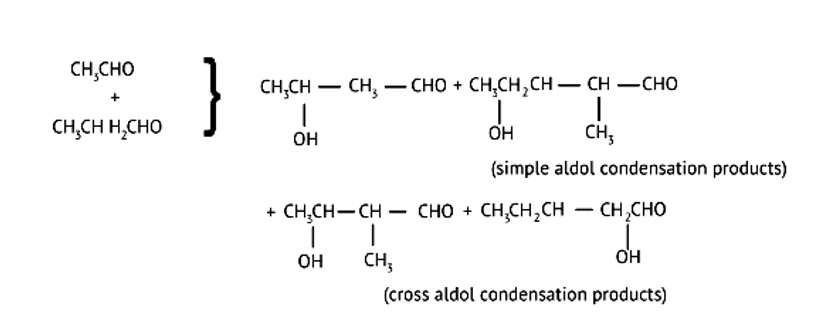
It's referred to as cross Aldol Condensation.Cross aldol condensation occurs when two separate aldehydes or ketones combine to form a single molecule.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
ClCH2COOH > FCH2COOH > C6H5CH2COOH > , CH3COOH > CH3CH2OH
When compared to other alcohols, CH3CH2OH is the least acidic. Because of the halogen, FCH2COOH is quite acidic. It is either an electron donating group or an electron receiving group in acidic strength. Acidic strength increases as the number of electron withdrawing groups increases. The acidic strength of electron donating groups diminishes.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The electrophile formed when benzene reacts with benzoyl chloride in the presence of anhydrous AlCl3 is benzoylinium cation, and the resultant product is benzophenone. Friedel Crafts acylation reaction is the name for this reaction.
New answer posted
6 months agoContributor-Level 10
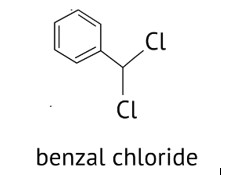
Benzal chloride can be made by chlorinating toluene in the presence of sunshine and then using the hydrolysis process to obtain benzaldehyde. This method can be used to make benzaldehyde in a commercial setting.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
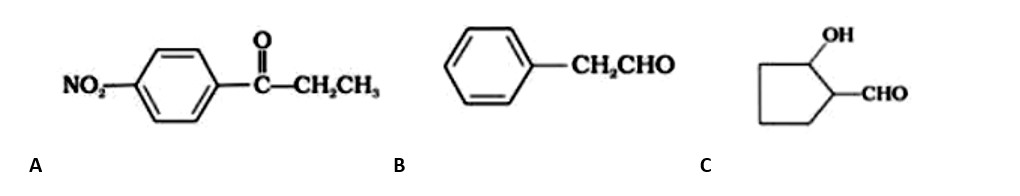
Ans:
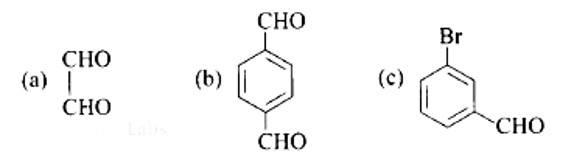
A: Ethane-1, 2-dial
B: Benzene-1, 4-carbaldehyde
C: 3-Bromobenzaldehyde
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
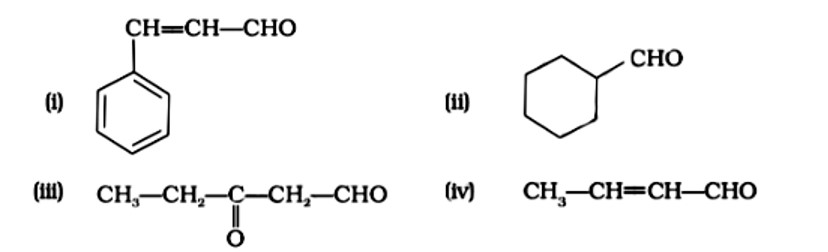
(i) 3-Phenylprop-2-ene-1-al.
(ii) Cyclohexanecarbaldehyde
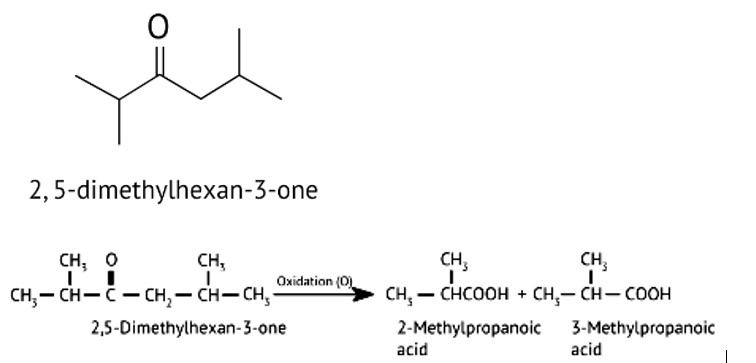
(iii) 3-Oxopentan-1-al
(iv) IUPAC name: But-2-enal
New answer posted
6 months agoContributor-Level 10
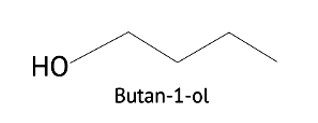
Butanol is a four-carbon aldehyde, with the aldehyde group on the fourth carbon. It has a functional group and a carbonyl group that interacts dipole-dipole. Because butanol possesses a polar O-H bond, it exhibits intermolecular H bonding, which is not feasible in butanal due to the lack of a polar bond. The boiling point of butanol is higher than that of butan-1-ol.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers