Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve
Get insights from 125 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Moles of chlorine in the given compound = Moles of chlorine in AgCl
= moles of AgCl
Mass of chlorine =
= 0.098 g
New answer posted
6 months agoContributor-Level 10
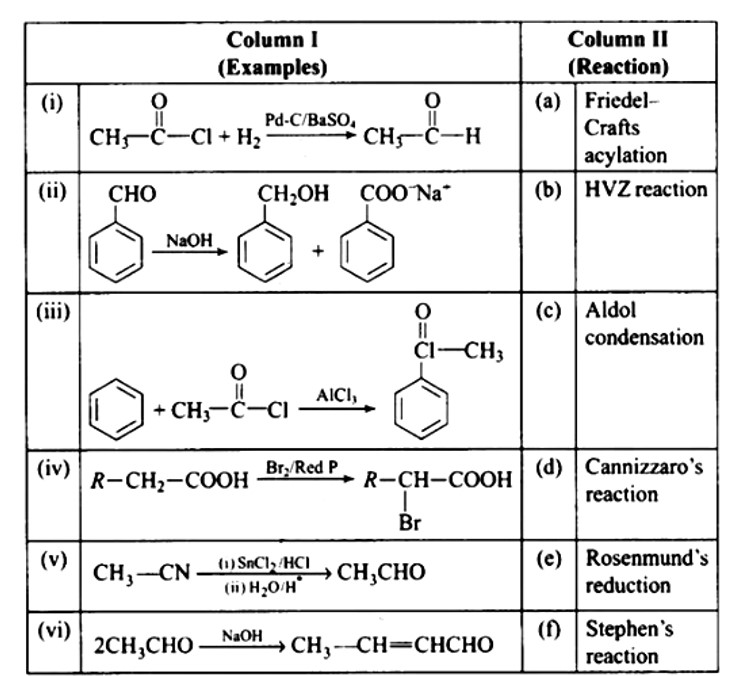
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) — (e); (ii) — (d); (iii) — (a); (iv) — (b); (v) — (f); (vi) — (c)
New answer posted
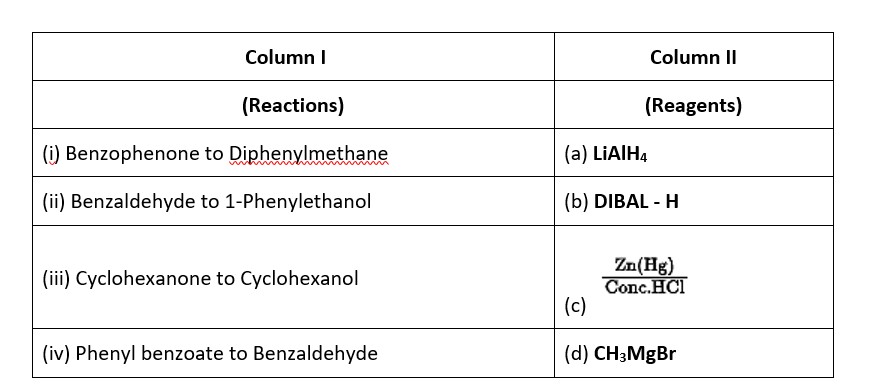
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) → (c); (ii) → (d); (iii) → (a); (iv) → (b).
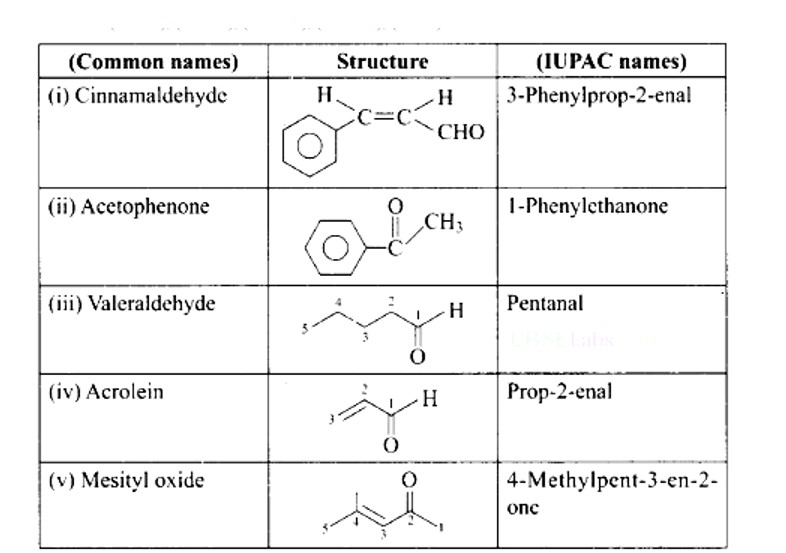
New answer posted
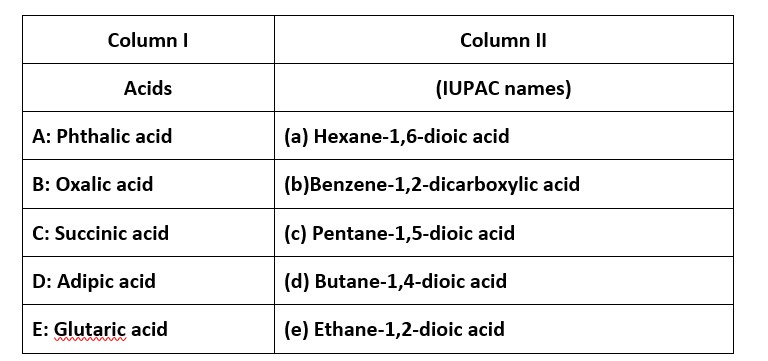
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) → (b); (ii) → (e); (iii) → (d); (iv) → (a); (v) → (c)
New answer posted
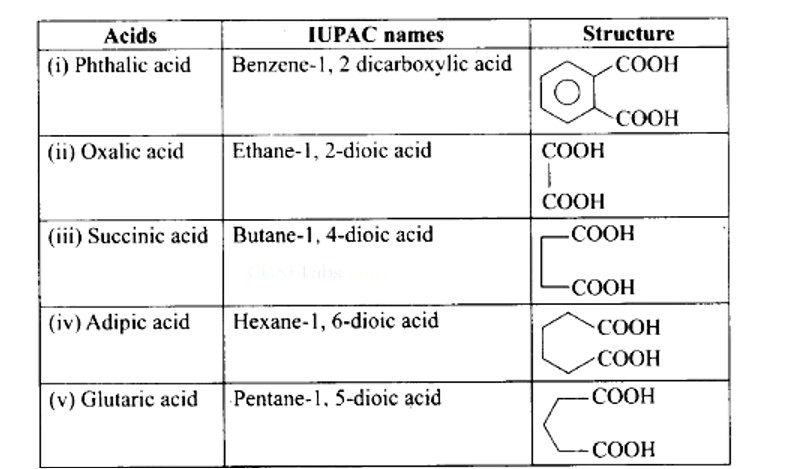
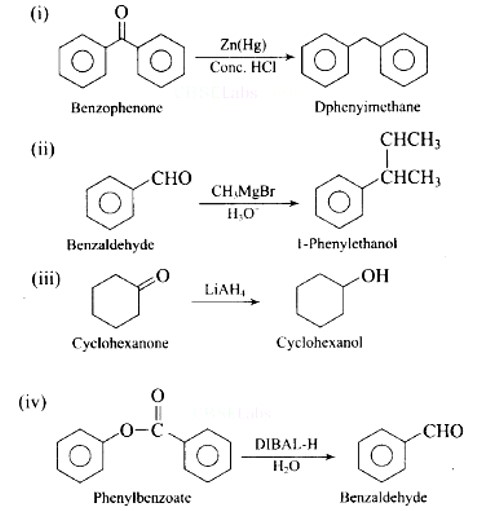
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) → (d); (ii) → (e); (iii) → (a); (iv) → (b); (v) → (c)
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: D
The silver mirror test can be used to determine Tollen's. [Ag (NH3)2] + OH - . Only aldehydes, not ketones, react with Tollen's reagent to create silver.
A silver mirror test is not given with this affirmative test.
The carbonyl group is present in both aldehyde and ketone.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: C
There is no alpha hydrogen atom in the Cannizaro process. Formaldehyde and aromatic aldehydes do not contain alpha hydrogen. It proceeds through the Cannizaro reaction. Formaldehyde is the most reactive of all aldehydes.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers