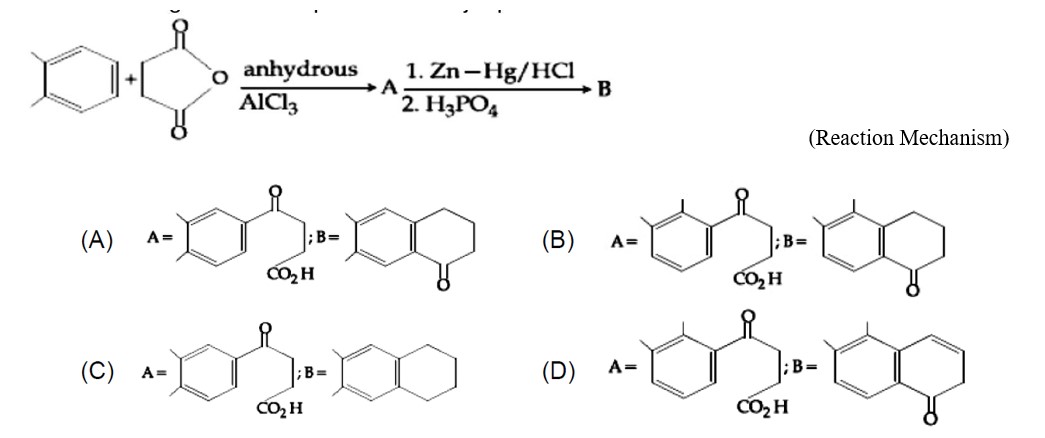
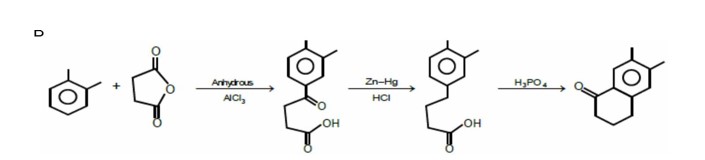
Chemistry Organic Chemistry
Get insights from 71 questions on Chemistry Organic Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Organic Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
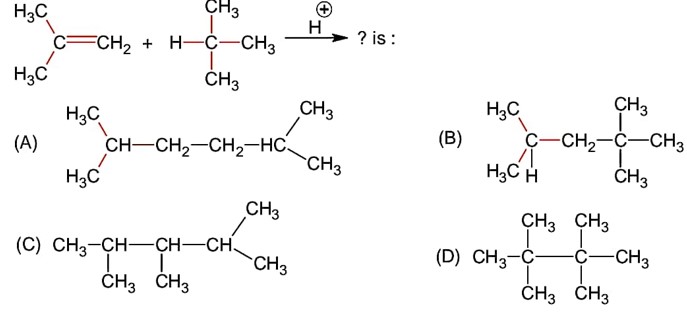
Molecular formula from calculation comes to be C3H6O i.e., it stands for the following compounds.
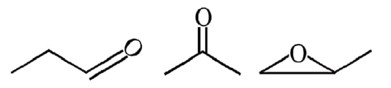
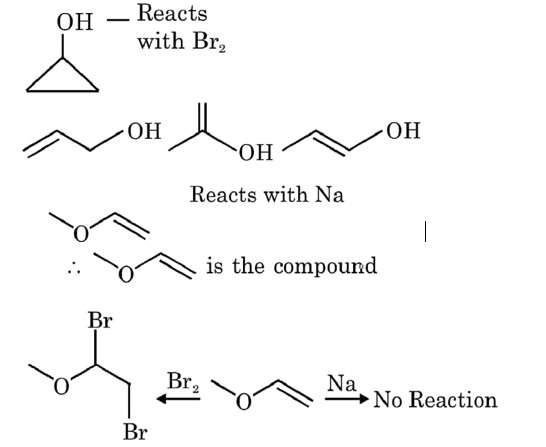
No addition reaction with Br2
VD * 2 = MM = 29 * 2 = 58
2.9 g of ether will combines with
Y = 9 and X = 8
Y - X = 9 - 8 = 1
New answer posted
4 months agoContributor-Level 9
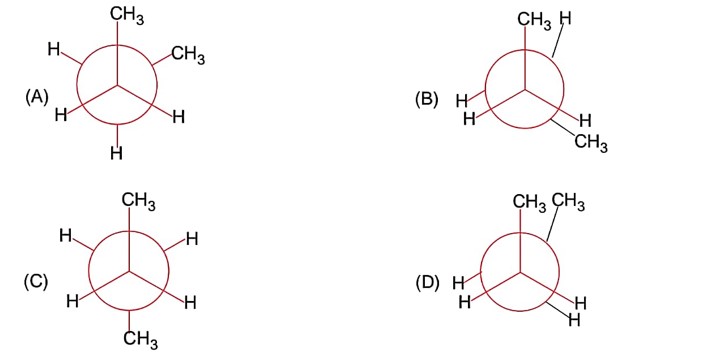
(A) and (C) are staggered conformation. In (A) dihedral angle is 60° and in (C) dihedral angle is 180°.
Dihedral angle is angle between two specified groups.
In (C) it has maximum angle.
New answer posted
4 months agoContributor-Level 9
Moles of carbon in organic compound = Moles of carbon in CO?
n_c = 420 / 44 moles
Mass of carbon in organic compound = (420 / 44) * 12 = 114.54 g
Moles of hydrogen in compound = 2 * moles of H? O
n_H = 2 * (210 / 18) moles
Mass of hydrogen = 2 * (210 / 18) g = 23.33 g
% of H = (23.33 / 750) * 100 = 3.11%
The nearest integer is 3.
New answer posted
4 months agoContributor-Level 9
Purification of a compound is independent of the physical state of the pure compound in chromatography techniques.
New answer posted
4 months agoContributor-Level 10
The partial pressure of dry N? is 758 - 14 = 744 mm Hg. Using the ideal gas law (PV=nRT), the moles of N? are calculated to be 1.25 * 10? ³ mol. This corresponds to 0.035 g of N? The percentage of nitrogen in the sample is (0.035 g / 0.1840 g) * 100, which is 18.96%.
Answer: 19 (Rounded)
New answer posted
4 months agoContributor-Level 10
The Lassaign's test is a qualitative analysis method used to detect nitrogen, sulfur, phosphorus, and halogens in an organic compound. Copper (II) oxide is used to detect carbon. In the sodium fusion extract, halides (X? ) precipitate with AgNO? , and sulfide (S²? ) precipitates as black PbS.
New answer posted
4 months agoContributor-Level 10
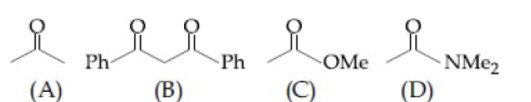
B is the most acidic as it is active methylene group. D is least acidic due to crossconjugation in conjugate base.
∴ Option 3 follows
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers