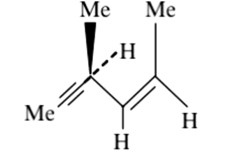
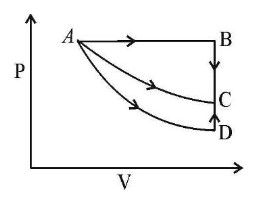
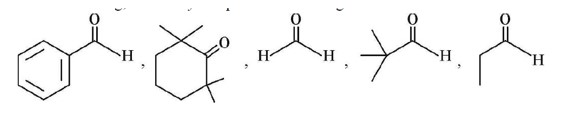
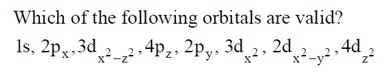Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoNew question posted
3 months agoNew question posted
3 months agoNew question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
The Pauli exclusion principle states that no two electrons in an atom can have the same four quantum numbers. In other words, only two electrons may exist in the same atomic orbital, and these electrons must have opposite spins. (a) and (f) violate the Pauli exclusion principle. Hund's rule states that the most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins. (b), (d) and (e) violate Hund's rule.
New question posted
3 months agoNew question posted
3 months agoNew question posted
3 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers