Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
2. 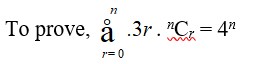
By binomial theorem,
(a + b)n = nC0 (a)n (b)0 + nC1 (a)n–1 (b)1 + …………. + nCr (a)n–r (b)r + …………… + nCn (a)n–n (b)n
Where, b0 = 1 = an–n
So, (a + b)n = nCr (a)n–r (b)r
Putting a = 1 and b = 3 such that a + b = 4, we can rewrite the above equation as
(1 + 3)n = nCr (1)n–r.3r
=>4n = .nCr
Hence proved.
New answer posted
7 months agoContributor-Level 10
1. For a number x to be divisible by y, we can write x as a factor of y i.e., x = ky where k is some natural number. Thus in order for 9n+1 – 8n – 9 to be divisible by 64 we need to show that 9n+1 – 8n – 9 = 64k where k is some natural number.
We have, by binomial theorem
(1 + a)m = mC0 + mC1 (a) + mC2 (a)2 + mC3 (a)3 + ………… + mCm (a)m
Putting, a = 8 and m = n + 1
(1 + 8)n+1 = n+1C0 + n+1C1.8 + n+1C2.82 + n+1C3.83 + ……. + n+1Cn+1. (8)n+1
=> 9n+1= 1 + (n + 1)8 + 82* [n+1C2 + n+1C3.8 + ………. + n+1Cn+1. (8)n+1–2] [since, n+1C0 = 1, n+1C1= n + 1]
=> 9n+1 = 1 + 8n + 8 + 64 * [n+1C2 + n+1C3.8 + ……�
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
24. Let P (n) be the statement “ 2n+7< (n+3)2
ofn=1
P (1): 2
9<16 which is true. This P (1) is true.
Suppose P (k) is true.
P (k)= 2k+7< (k+3)2 . (1)
Lets prove that P (k +1) is also true.
“ 2 (k + 1) + 7 < (k + 4)2=k2+ 8k + 16”
P (k +1) = 2 (k +1) +7 = (2k +7) +2
< (k +3)2+ 2 (Using 1)
= k2+ 9 + 6k +2 = k2+6k +11
Adding and subtracting (2k + k) in the R. H. S.
for all
is true.
By the principle of mathematical induction, P (n)is true for all n N.
New answer posted
7 months agoContributor-Level 10
23. Let
Put n= 1,
is a multiple of 27.
Which is true.
Assume that P(k) is true for some natural no. k.
P(k)= be a multiple of 27
i.e,
(1)
We want to prove that P(k+1) is also true.
Now,
(Using 1)
is true when P(k) is true.
Hence, by P.M.I. P(n) is true for every positive integer n.
New answer posted
7 months agoContributor-Level 10
22. Let P(n): is divisible by 8
put n= 1,
P(1):
34 – 8 – 9 = 81– 17 = 64= is divisible by 8
Which is true.
Assume that P(k) is true for some natural numbers k.
i.e, be divisible by 8
where,a
(1)
We want to prove thatP(k+ 1) is true.
is divisible by 8, is also true.
Now,
=
3(2k +2). 32 8k 17
(Using 1)
= 72a + 64k+ 64 = 8(9a + 8k + 8)
= 8b, where b = 9a + 8b + 8
32k + 4– 8(k+1) – 9 is divisible by 8.
P(k+1) is true when P(k) is true. Hence, By P.M.I. P(n) is true for all positive integer n.
New answer posted
7 months agoContributor-Level 10
21. Let
Assume that P(k) is true for some natural no. k
i.e.
Now, let us prove P(k +1) is true.
Hence, by P.M.I. P(n) is true for all natural number i.e.
New answer posted
7 months agoContributor-Level 10
20. LetP(n):
Putting n = 1
Which is true. Thus, P(1) is true.
Let us assume that P(k) is true for some natural no. k.
P(k)=
we want to prove that P(k +1) is true.
=1100a
11b where b= (100a
is true when p(k) is true.
Hence by P.M.I. P(n) is true for every positive integer.
New answer posted
7 months agoContributor-Level 10
19. We can write the given statement as
P (n): n (n +1) (n+5), which is multiple of 3.
If n= 1, we get
P (1)=1 (1+1) (1+5)=12, which is a multiple of 3 which is true.
Consider P (k) be true for some positive integer k
k (k+1) (k+ 5) is a multiple of 3
k (k+1) (k+5)= 3 m, where
Now, let us prove that P (k + 1) is true
Here,
(k+ 1) { (k+1)+ 1} { (k+1)+ 5}
We can write it as
= (k +1) (k+ 2) { (k + 5) + 1}
By Multiplying the terms.
By eqn. (1)
= 3m + 2 (k + 1) (k + 5) + (k + 1) (k + 2)
= 3m + (k + 1) {2 (k + 5) + (k +2)}
= 3m + (k + 1) {2k + 10 +k + 2}
= 3m + (k + 1) (3k +12)
= 3m + 3 (k + 1) (k+ 4)
=3 {m + (k + 1) (k + 4)}
3
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers