Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
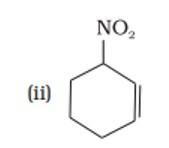
18. In this structure i.e., 3-nitrocyclohex-l-en (Carbon atoms of the ring are numbered in such a way that double bonded carbon gets the lowest number followed by the nitro group -NO2.
New answer posted
7 months agoContributor-Level 10
24. In this structure i.e., 3-ethyl, 4-methylhept-5-en-2-one. (the longest carbon chain is selected in such a way that the functional group > C = O gets the lowest possible locant.
New answer posted
7 months agoContributor-Level 10
23. Whenever an organic compound contains both N and S, then on fusion with Na metal compound gives NaSCN or NaCN and Na2S depending on the quantity of Na metal. If Na metal is less, then only NaSCN is formed. In that case L.E on treating with FeSO4 and H2SO4 gives red colour due to the formation of ferric thiocyanide Fe (SCN)3. In case of NaCN, L.E on treating with FeSO4 and H2SO4gives Prussian blue colour.
Chemical reactions: Fe2+ →Fe3+ (Oxidation)
Fe3+ + 3NaSCN →Fe (SCN)3 + 3Na+
&nb
New answer posted
7 months agoContributor-Level 10
22. Four possible carbocations can be obtained from 2- methyl butane are

Generally, order of stability follows the order-
1° carbocation < 2 carbocation < 3 carbocation
Order of increasing stability of the given structures is-
I < IV < II < III.
New answer posted
7 months agoContributor-Level 10
21. Triphenylmethyl cation is found to be very much stable as this positive charge on methyl carbon is delocalized in three phenyl rings. In each phenyl ring, positive charge is developed on 2 ortho position and para position, i.e., three resonating structures. Total resonating structures given by triphenylmethyl cation are nine. Hence, it is very stable. These structures can be shown as.

New answer posted
7 months agoContributor-Level 10
20. The structure A is more stable than structure B. This is because carbocation A is more Planar and? electrons from the ring shift to the side group and are stabilized by resonance while structure B is non-planar and does not undergo resonance. Also, double bond is more stable within the ring as compared to the side chain.
New answer posted
7 months agoContributor-Level 10
19. The resonating structures can be drawn as-

In structure I, CH2 group has the positive charge which means octet is not completed, but in structure II, both the carbon atoms and oxygen atom have complete octet hence, more stable.
New answer posted
7 months agoContributor-Level 10
18. If a liquid compound decomposes at its boiling point but is steam volatile and insoluble in water and stable at low pressure. Then steam distillation method can be used for its purification.
Note : Answer the questions 35 to 38 on the basis of information given below:
“Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged carbon atom involvement of neighbouring groups in hyperconjugation and resonance.”
New answer posted
7 months agoContributor-Level 10
17. In DNA and RNA, nitrogen is present in heterocyclic base and also present in the ring but not as a substituent. Therefore, nitrogen present in the ring cannot be converted into (NH4 )2SO4. Hence, it cannot be estimated by Kjeldahl method.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers