Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
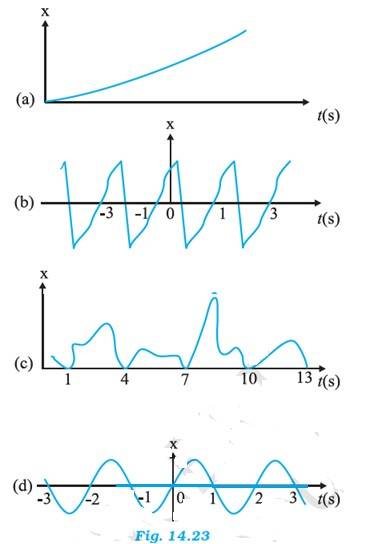
(a) It is not a periodic motion. It represents a unidirectional, linear uniform motion. There is no repetition of motion
(b) In this case, the motion of the particle repeats itself after 2 s. Hence, it is a periodic motion, having a period of 2 s
(c) It is not a periodic motion. This is because the particle repeats the motion in one position only. For a periodic motion, the entire motion of the particle must be repeated in equal intervals of time
(d) In this case, the motion of the particle repeats itself after 2 s. Hence, it is periodic motion, having a period of 2 s
New answer posted
8 months agoContributor-Level 10
(a) During its rotation about its axis, earth comes to the same position again and again in equal intervals of time. Hence it is a periodic motion. However, this motion is not simple harmonic, as earth does not have to and fro motion about its axis.
(b) An oscillating mercury column in a U-tube is simple harmonic. This is because the mercury moves to and fro on the same path, about the fixed position, with a certain period of time.
(c) The ball moves to and fro about the lowermost point of the bowl when released. Also the ball comes back to its initial position in the same period of time, again and again. Hence, its motion
New answer posted
8 months agoContributor-Level 10
(a) The swimmer's motion is not periodic. The motion of the swimmer between the banks of a river is back and forth. However, it does not have a definite period. This is because the time taken by the swimmer during his back and forth journey may not be the same.
(b) The motion of a freely-suspended magnet, if displaced from its N-S direction and released, is periodic. This is because the magnet oscillates about its position with a definite period of time.
(c) When a hydrogen molecule rotates about its centre of mass, it comes to the same position again and again, after an equal interval of time. Such motion is periodic.
&nbs
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
The potential associated with each electrode is known as electrode potential. If the concentration of each species taking part in the electrode reaction is unity (if any gas appears in the electrode reaction, it is confined to 1 atmospheric pressure) and further there action is carried out at 298K, then the potential of each electrode is said to be the Standard Electrode Potential. By convention, the standard electrode potential (E? ) of hydrogen electrode is 0.00 volts. The electrode potential value for each electrode process is a measure of the relative tendency of the active species in the process to remainin the oxidised/reduced fo
New answer posted
8 months agoContributor-Level 10
Two methods are used to balance chemical equations for redox processes. One of these methods is based on the change in the oxidation number of reducing agent and the oxidising agent (i.e. oxidation number method) and the other method is based on splitting the redox reaction into two half reactions — one involving oxidation and the other involving reduction (half reaction method). Both these methods are in use and the choice of their use rests with the individual using them.
New answer posted
8 months agoContributor-Level 10
(a) Combination reactions (b) Decomposition reactions (c) displacement reactions (d) Disproportionation reactions.
New answer posted
8 months agoContributor-Level 10
Electrochemical series is the series of elements in which elements are arranged in decreasing order of their reduction potential.
Reducing power goes on increasing whereas oxidising power goes on decreasing down the series.
New answer posted
8 months agoContributor-Level 10
Electrochemical cell is a device in which the redox reaction is carried indirectly and the decrease in free energy appears as electrical energy.
At cathode there is gain of electrons.
At anode there is loss of electrons.
In electrochemical cell anode is written on L.H.S while cathode is written on R.H.S.
New answer posted
8 months agoContributor-Level 10
(b) Oxidation involves gain of one or more electrons by a species during a reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers