Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
3.6 The speed of the car = 126 kmph = (126 * 1000/3600) m/sec = 35 m/s
From the relation V2 = U2 + 2fs, where Final velocity V = 0, U = 35 m/s, s = 200m, we get retardation, f = -U2/2s= 3.06 m2/s
From the relation V = U – ft, we get t = U/f = 35/3.06 = 11.42 s.
New answer posted
8 months agoContributor-Level 10
3.5 The speed of the jet plane, Vj = 500 kmph. If the speed of the product of combustion is Vpc, then V pc = Vpc-Vj = -1500 kmph (relative to the jet plane)
Speed of the combustion product relative to the observer on the ground,
Vpc = -1500 + Vj = -1500+500 = -1000 kmph
New answer posted
8 months agoContributor-Level 10
3.4
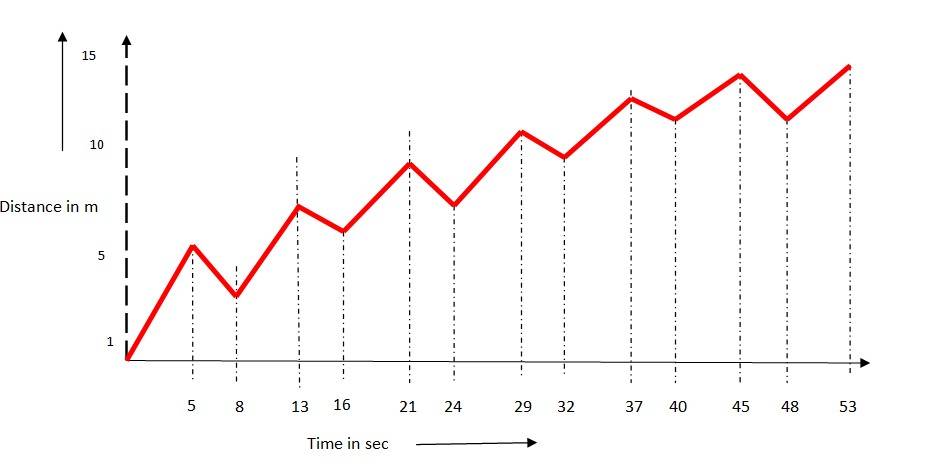
The time taken for each step is 1s, he covers 5 m in 5 secs and goes backward by 3 m in 3 secs.
So in 8 s he covers 2 m. To cover 13m distance he needs to complete 8 m (13-5). To cover 8m, he will require 8 * 4 = 32 s. To fall in a pit, he needs to move another 5m in 5s.
So total time taken = 32 +5 = 37 s
Let's have a look at how we came to this answer.
We have to calculate how long it will take for the drunkard to fall into a pit located 13 metres from his starting point.
In each cycle of movement, the drunkard takes 5 steps forward and 3 steps backward. We calculate the net displacement per cycle.
Time for one cycle = 5 seco
New answer posted
8 months agoContributor-Level 10
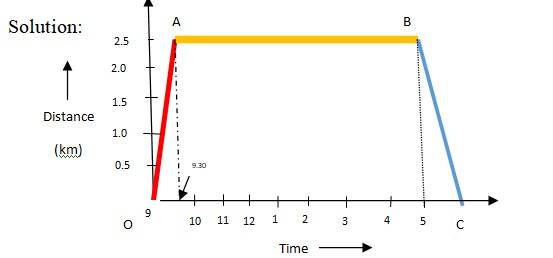
3.3 Office distance = 2.5 km
Walking speed = 5 kph
Time taken to reach office = 5/2.5 = 0.5 h
OA is the path to reach office
AB is the duration of office stay
Auto speed = 25 kph
Time taken by auto = 2.5/25 h = 6 minutes
BC is the path for returning
On the position-time graph, this is a straight line sloping downwards. It starts at the point representing 5:00 PM and 2.5 km, and ends at 5:06 PM at 0 km. It tells us that the woman has arrived home.
The slope of this line represents instantaneous velocity. Because the return trip is by the auto at a much higher speed (25 km/h), the slope of line BC is significantly steeper than the line
New answer posted
8 months agoContributor-Level 10
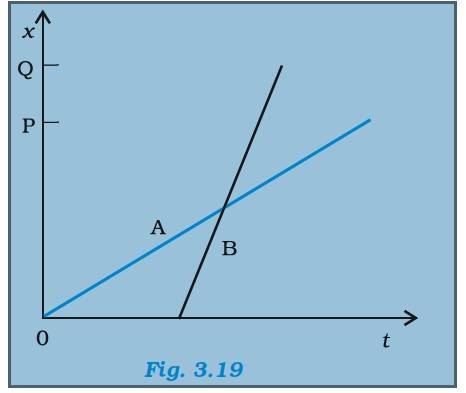
3.2
(a) A lives closer to the school than B ( OP
(b) A starts earlier than B from school. From the graph, A starts from O at time 0 whereas B starts after some finite value t
(c) B walks faster than as the slope for B is steeper than A
(d) Both B and A reaches home at the same time
(e) At the intersection of the two lines, it is evident that B overtakes A once
New answer posted
8 months agoContributor-Level 10
This question belongs to the Motion in a straight line chapter that we will be solving this with explanation.
(a) A railway carriage moving without jerks between two stations
Whenever a railway carriage moves between two stations smoothly without jerks, it is considered as a point object. This is due to the fact that distance between the two stations is larger as compared to the size of carriage. However, we are interested in the overall motion instead of the movement of specific parts of carriage.
(b) A monkey sitting on top of a man cycling smoothly on a circular track
Here, both monkey as well as the cyclist are considered as a single p
New answer posted
8 months agoContributor-Level 10
2.32
Distance of the moon from Earth = 3.84 * 108 m
Distance of the Sun from Earth = 1.496 * 1011 m
Sun's diameter = 1.39 * 109 m
Sun's angular diameter = 1920° = 1920 * 4.85 * 10-6 rad = 9.3 * 10-3 rad
During total Solar eclipse, the moon completely covers the Sun, then the angular diameter of both Sun and the
Moon will be equal
So the angular diameter of moonΘ = 9.3 * 10-3 rad, distance d of moon from Earth = 3.84 * 108 m and
diameter of the moon = angular diameter * distance
So the approximate diameter of the Moon = 9.3 * 10-3 * 3.84 * 108 m = 35.712 * 105 m
New answer posted
8 months agoContributor-Level 10
2.31
Speed of light = 3 * 108 m/s, Time taken = 3 billion years = 3 * 109 years = 3 * 365 * 24 * 60 * 60 * 109 s
The distance = speed x time = 3 * 108 * 3 * 365 * 24 * 60 * 60 * 109 m
= 2.838 * 1025 m
New answer posted
8 months agoContributor-Level 10
2.30
Speed of the sound in water = 1450 m s–1
Time taken from generation + reflection = 2t = 77 s
From the relation s = ut, where s is the distance of the enemy ship, we get
s = 1450 * 77/2 m = 55825 m = 55.8 km
New answer posted
8 months agoContributor-Level 10
2.29 Time taken by the laser beam after reflection = 2.56 s
The speed of laser = 3 * 108 m/s
If the distance between Earth and the Moon is d, the distance covered by laser beam is 2d
From the relation distance = speed * time, we get
d = (2.56 * 3 * 108)/2 = 3.84 * 108 m
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers