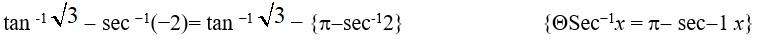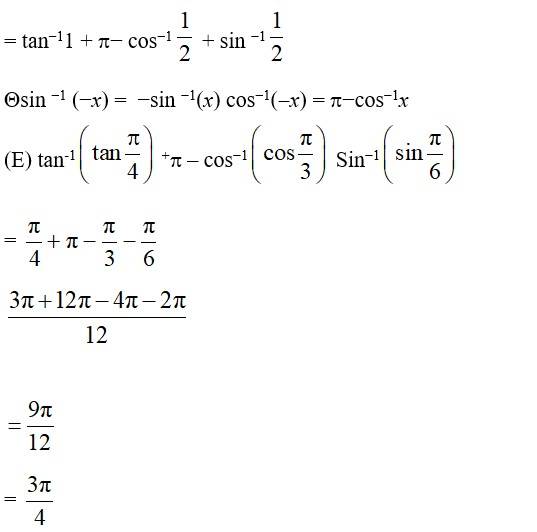Class 12th
Get insights from 11.8k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
We know that,
cos3θ= 4cos3θ - 3cosθ
Letx = cosθ Then θ = cos-1x. We have,
Cos3 (cos-1x) = 4x3-3x
3cos-1x = cos-1 (4x3- 3x)
Hence Proved
New answer posted
8 months agoContributor-Level 10
We know that.
sin 3θ =3 sin θ 4sin3θ (identity).
(E) Let x = sinθ. Then, sin −1x=θ . We have,
Sin3 (sin −1x) = 3x−4x3
3sin −1x =sin-1 (3x−4x3)
Hence proved.
New answer posted
8 months agoContributor-Level 10
Given, Sin−1x=y.
(E) We know that the principal value branch of Sin−1 is
Hence, ≤ y ≤
Option B is correct.
New answer posted
8 months agoContributor-Level 10
Let cos -1 =y Then cos y = = − cos
cos
= cos
= cos
(E) We know that the range of principal value
branch of cos−1 is [0, ] and cos =
Principal value of cos−1 is
New answer posted
8 months agoContributor-Level 10
Let sin−1 =y. Then, sin y=-
We know that the range of principal value branch of sin−1 is
and sin−1
=
Principal value of sin−1
New answer posted
8 months agoContributor-Level 10
4.39 Given, k2 = 4k1, T1 = 293K and T2 = 313K
We know that from the Arrhenius equation, we obtain
On solving, we get,
Ea = 58263.33 J mol-1 or 58.26 kJ mol-1
New answer posted
8 months agoContributor-Level 10
4.38 We know, time t = (2.303/k) * log ( [R]0/ [R])
Where, k- rate constant
[R]0-Initial concentration
[R]-Concentration at time 't'
At 298K, If 10% is completed, then 90% is remaining. t = (2.303/k) * log ( [R]0/0.9 [R]0)
t = (2.303/k) * log (1/0.9) t = 0.1054 / k
At temperature 308K, 25% is completed, 75% is remaining t' = (2.303/k') * log ( [R]0/0.75 [R]0)
t' = (2.303/k') * log (1/0.75) t' = 2.2877 / k'
But, t = t'
0.1054 / k = 2.2877 / k' / k = 2.7296
From Arrhenius equation, we obtain log k2/k1 = (Ea / 2.303 R) * (T2 - T1) / T1T2
Substituting the values,
Ea = 76640.09 J mol-1 or 76.64 kJ mol-1 We know, log k = log A –Ea/RT
Log k =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers