Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
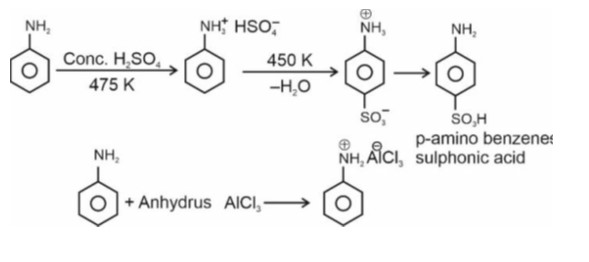
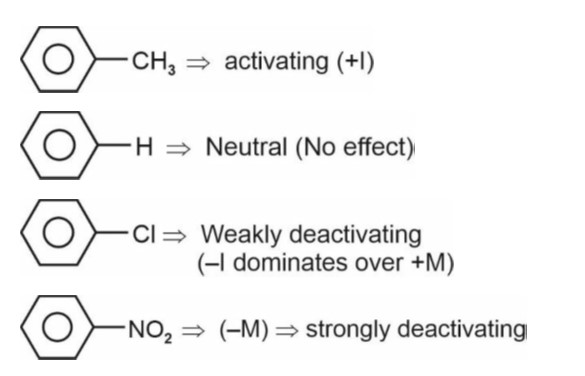
p-amino benzene sulphonic acid contains both N and S, so it gives blood red colour with Lassaigne's test.
New answer posted
3 months agoContributor-Level 10
A, B and C are correct.
Mn2O7 is a green oil at room temperature.
V2O4 react with acids to give VO2+.
CrO is Basic and CrO3 is acidic.
V2O5 react with acids as well as alkali.
New answer posted
3 months agoContributor-Level 10
(a) [Cr (H2O)6]+3 → Cr+3 →
(b) [Fe (H2O)6]+3 → Fe3+ →
(c) [Ni (H2O)6]+2 → Ni2+ →
(d) [V (H2O)6]+3 → V3+ →
New answer posted
3 months agoContributor-Level 10
Reducing character increases from NH3 to BiH3.
Group 15 oxides of type E2O3 and E2O5 are not always basic.
New answer posted
3 months agoContributor-Level 10
Ni2+ : 4s03d8 (No pairing with Cl–)
[Ni (CO)4] : 4s03d10 (diamagnetic)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers