Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Invertase Cane sugar to glucose and fructose
Zymase Glucose to ethanol
Diastase Starch to Maltose
Maltase Maltose to glucose
New answer posted
6 months agoContributor-Level 10
HOCl produce in the stratospheric cloud, by the hydrolysis reaction of ClONO2.
New answer posted
6 months agoContributor-Level 10
In 4d orbital, n = 4 and
Radial nodes =
Radial nodes = 4 – 2 – 1 = 1
And angular nodes,
New answer posted
6 months agoContributor-Level 10
Due to H- bond in water, it has high melting point and melting point of other hydrides of the group are depending upon the molecular weight.
New answer posted
6 months agoContributor-Level 10
Due to high crystallity Be has the highest M.P.
Be = 1560 K
Mg = 925 K
Ca = 1120 K
Sr = 1062 K
New answer posted
6 months agoContributor-Level 10
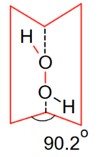
During the electrolysis of dilute H2SO4
In the solid form of dihedral angle is equal to 90.2°.
New answer posted
6 months agoContributor-Level 10
According to Ellingham diagram, the metal oxide with lower is more stable than metal oxide of higher at same temperature.
New answer posted
6 months agoContributor-Level 10
is the temperature Co-efficient of cell. The cell having less variation of EMF, with respect to temperature have high efficiency.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers