Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Ans: Correct Option: (iii)
(i)The electrode potential of Cu2+/Cu is negative. The electrode potential of 2H+/H2 is 0.00V. Therefore, the assertion is correct and the reason is incorrect and the reason is not a correct explanation of assertion. Therefore, this option is incorrect
(ii) E Cu2+/Cu is not negative so the reason is incorrect. Therefore, this option is incorrect
(iii) The electrode potential of Cu2+/Cu is negative. The electrode potential of 2H+/H2 is 0.00V. Therefore, the assertion is correct and the reason is incorrect. Therefore, this opt
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (b) ; (ii)- (a) ; (iii)- (d); (iv)- (c)
The rate rule was discovered experimentally and can be used to predict the reaction rate and reactant concentrations.
The rate constant is the proportionality constant that describes the relationship between the molar concentration of the reactants and the rate of a chemical reaction.
The rate constant (k) of a reaction is proportional to its temperature, i.e., for a given reaction at a given temperature, the rate constant (k) is constant.
The order of a reaction is a quantity that has been empirically determined. As a result
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) F2 is a non-metal which is the best oxidizing agent since the standard reduction potential of F2 is 2.87V.
(ii)Liis a metal and the strongest reducing agent because the standard reduction potential of Li is −3.05V.
(iii)Au3+ is a metal ion which is an oxidizing agent as standard reduction potential of Au3+ is 1.40V.
(iv) Br−is an anion that can be oxidized by Au3+ as standard reduction potential of Au3+ is 1.40V which is more than that of Br− which is E Br2/Br−0=1.09V.
(v)Auis an unreactive metal
(vi)Li+ is a metal ion having the least value
New answer posted
7 months agoContributor-Level 10
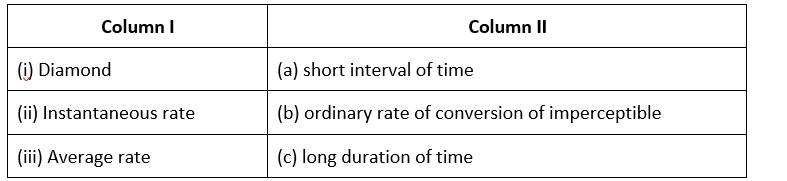
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (b) ; (ii)- (c) ; (iii)- (a)
The rate of conversion in diamond is normally imperceptible.
Long-term rate at a moment's notice
The average rate is only for a short time.
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i)The reaction occurring at Lechlanche cells are
At anode: Zn→Zn2+ + 2e−
At cathode: MnO2 + NH4+ + e−→MnO (OH)+NH3
(ii)Ni−Cd is rechargeable. So it has a longer lifetime.
(iii) Energy in the fuel cell is due to the combustion process. It converts combustion energy into electrical energy 2H2 + O2→2H2O
(iv) Mercury cells don't involve any solution and are used in hearing aids.
Hence, the answer is:
(i)-d; (ii)-c; (iii)-a, e; (iv)-b
New answer posted
7 months agoContributor-Level 10
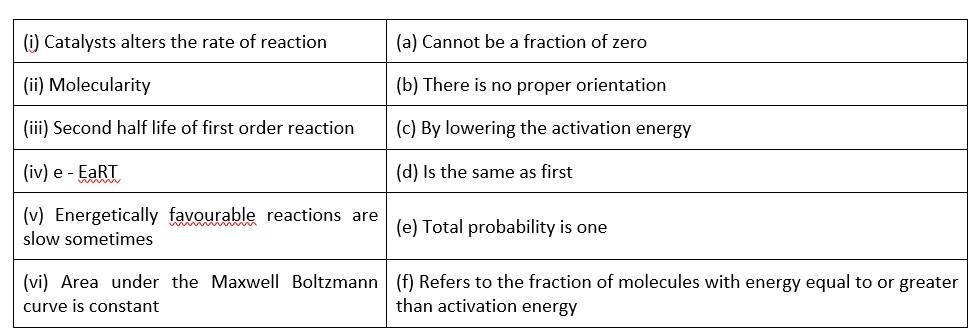
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (c) ; (ii)- (a) ; (iii)- (d); (iv)- (f); (v)- (b); (vi)- (b)
A catalyst can influence the rate of a process by lowering the activation energy
When it comes to molecularity, there can't be a fraction or a zero.
The second half life of a first order reaction is the same as the first.
Activation energy refers to the percentage of molecules with an energy equal to or greater than activation energy.
Correct orientation is not always present when it comes to energetically favourable activities.
Because the area under the Maxwell Boltzmann curve is constant, the total probability
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i)Conductivity k is defined as k =
(ii)Molar conductivity is given by Λm =
(iii)Degree of dissociation is given by ? =
(iv)Charge is the product of current and time = I
Hence, the answer is:
(i)-d; (ii)-c; (iii)-b; (iv)-a
New answer posted
7 months agoContributor-Level 10
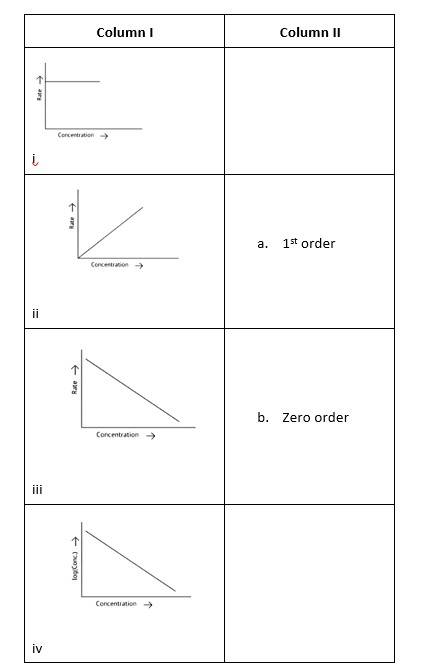
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (a) ; (ii)- (b) ; (iii)- (b); (iv)- (a)
A zero-order reaction is one in which the reactant concentrations do not change over time and the rate of concentration remains constant.
A first-order reaction is one in which the rate of the reaction is linearly proportional to the concentration of only one ingredient. In other terms, a first-order reaction is a chemical reaction whose rate is determined by changes in only one of the reactants' concentration.
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) The cell reaction of a lead storage battery is as follows;
Pb + PbO2 + 2H2SO4→2H2SO4 + 2H2O
At cathode:PbO2 (s)+SO42− (aq)+2e−→2PbSO4 (s) + 2H2O (l)
At anode:Pb (s)+SO42− (aq)→PbSO4 (s)+2e−
Therefore Pb is the anode and PbO2 is cathode
(ii)Mercury cell doesn't contain ions so it gives steady potential.
(iii)Fuel cells have maximum efficiency as they produce energy due to the combustion reaction of fuel.
(iv)Rusting is prevented by galvanization.
Hence, the answer is:
(i)-d ; (ii)-c ; (iii)-a ; (iv)-b
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i)On dilution, the number of ions per unit volume increases so the molar conductivity increases with dilution.
(ii) E cell is an intensive property as it is not dependent on the amount or mass of the substance.
(iii) k is conductivity or be precise specific conductivity which depends on the number of ions
(iv) ΔrGcell is an extensive property as it depends on the amount of substance or number of particles in the solution.
Hence, the answer is:
(i)-d ; (ii)a ; (iii)-b ; (iv)-c
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers