Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, iii)
Electrolysis of copper sulfate solution is as follows
CuSO4 ? Cu2+ + SO42−
H2O? H+ + OH−
At the cathode, the reaction goes this way,
Cu2+ + 2e− → Cu; E? Cell = 0.34V
H2O− → H2 E? Cell = 0.00V
At the anode, the reaction goes this way,
2SO42- + 2e−→S2O82− E? Cell =1.96V
2H2O→O2 + 4H+ + 4e− E? Cell =1.23V
The reaction will lower the value of E? Cell is preferred at anode so the second reaction is feasible.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, iii)
Kohlrausch law of states that limiting molar conductivity of any salt species is equal to the sum of the limiting molar conductivity of cations and anions of the electrolyte
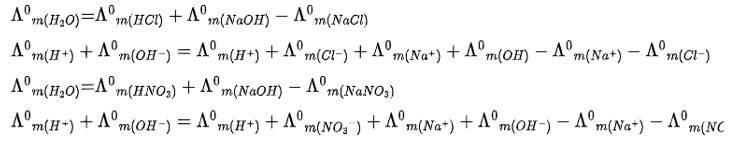
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and C
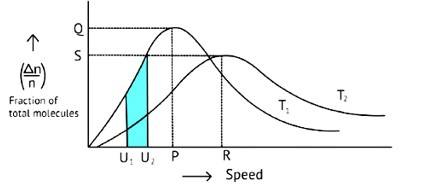
The Maxwell Boltzmann distribution, often known as the Gibbs distribution, is a measure that expresses the likelihood of a system being in each state as a function of the energy of that state.
Look at the graph.
T2 > T1
The fraction of molecules falls as the temperature rises because the area under the curve shrinks.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, ii)
Conductivity is due to the movement of ions in the solution. The conductivity of ions depends on the following factors:
(i) nature of electrolyte added
(ii) size of ion produced
(iii) concentration of electrolyte
(iv) nature of the solvent
(v) temperature
Distance between electrodes does not affect the conductivity of an electrolytic solution.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (ii, iii)
At equilibrium, ?G = -2.303 RT
-nFE = -2.303 RT
E =
For Daniel cell, n=2
E =
At equilibrium, E = 1.1V
1.1V =
(i) Since this option is incorrect
(ii) As derived 1.1V =
(iii) As derived so this option is correct
(iv) As ,so this option is incorrect
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
Activation Energy is the amount of energy released when reactant molecules collide and form an activated complex. When energy is released, the complex decomposes into a product.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, iii)
In the electrolysis of sulphuric acid, the following reactions occur
2SO42− (aq)→S2O82− (aq) + 2e− E? Cell =1.96V
2H2O (l)→O2 (g)+4H+ (aq) + 4e− E? Cell =1.23V
The reaction will lower the value of E? Cell is preferred at anode so the second reaction is feasible.
H+ + e− → H2 E? Cell = 0.00V
At the cathode, reduction of water occurs. Therefore, in dilute sulphuric acid solution, hydrogen will be reduced at the cathode.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A, C and D
The pressure in this reaction is extremely high, and it becomes independent of the ammonia concentration. The metal surface becomes saturated with gas molecules when the rate of reaction=Rate constant. The rate of a zero-order reaction is independent of the concentration of reactants in the reaction.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (ii, iv)
The lesser the E? value of the redox couple higher is the reducing power
For Cu2+ + 2e−→Cu; E? =0.34V
For 2H+ + 2e−→H2 E? =0.00V
Since the second redox couple has less standard reduction potential than the first so it can be concluded that the redox couple is a stronger oxidizing agent than H+/H2 and copper cannot displace H2 from acid.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
A complex reaction is one that does not happen in a single step.
The total number of molecules involved in the slowest step of the reaction determines the overall reaction rate.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
