Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
(a) Cumene is the beginning element for the industrial production of phenol.
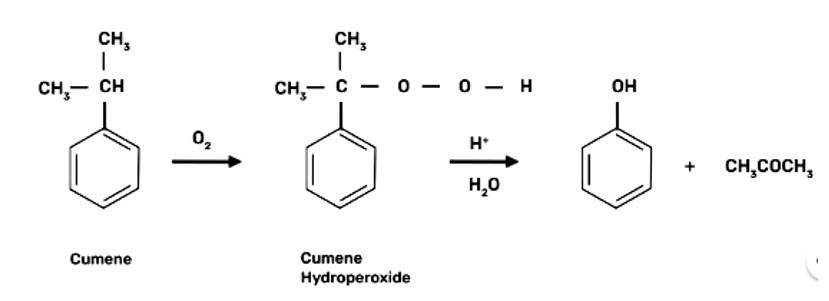
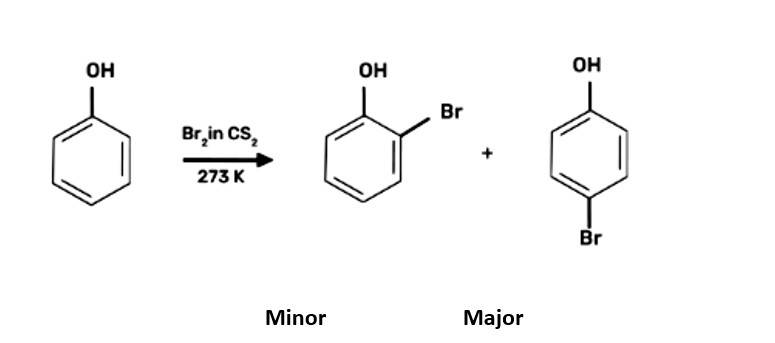
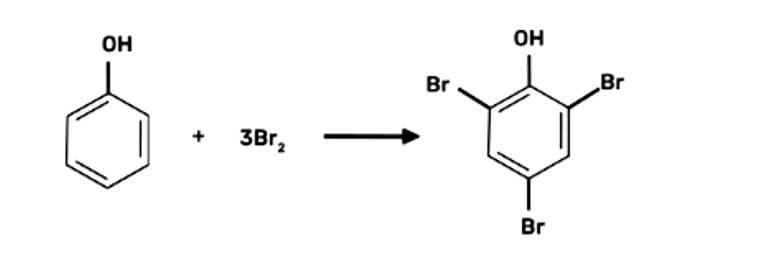
When bromine water is used to treat phenol. As a whitish precipitate, 2,4,6-tribromophenol is formed
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
In case of anisole, methylphenyl oxonium ion, 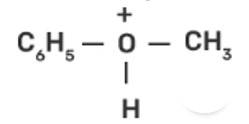
New answer posted
7 months agoContributor-Level 9
After the NEET results, Indian students can make NSMU Russia application on the official wesbite of this university.
Indian students are required to clear 12th standard with a minimum of 50% in 12th class with Physics, Chemistry, Maths. A certain level of English and Russian is also required, however the students don't have to take IELTS, or TOEFL.
New answer posted
7 months agoContributor-Level 10
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
Correct option: C
A catalyst allows a chemical reaction to occur at a faster rate or under different conditions than it would otherwise. As a result, a catalyst affects the reaction's enthalpy change, or heat. As a result, in the presence of the reaction, the enthalpy does not vary, i.e., it remains constant, and no heat is produced or absorbed.
New answer posted
7 months agoContributor-Level 10
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
Correct option: B
The Arrhenius equation can be used to calculate the activation energy of a chemical process. At two temperatures, this determines the rate constants.
2.303log = =
New answer posted
7 months agoContributor-Level 10
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
Option C
Gibbs energy of reaction - Gibbs free energy is a single-valued combination of entropy and enthalpy. The direction of a chemical reaction at constant temperature and pressure is predicted by Gibbs free energy.
Enthalpy of reaction – When one mole of matter is converted by a chemical reaction under specified conditions, the enthalpy of reaction is the change that occurs in the system.
Activation energy of reaction – The lowest amount of energy necessary to activate molecules to a state where they can perform physical and chemical transformations is known
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Ans: Correct Option: (iv)
(i) Copper sulfate can't be stored in zinc vessels due to less reactive nature than zinc due to the negative standard reduction value of zinc.
So both assertion and reason are false. Therefore, this option is incorrect.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Ans: Correct Option: (ii)
(i) The Nernst equation can be written as follows
Ecell = E cell – log
Ecell = E cell + 0.059 logAg+
Here we observe that EAg+/Ag increases with increase in the concentration of Ag+ ions. It has a positive value.
Here both assertion and reason are correct .The reason is not a correct explanation of assertion. So this option is incorrect
(ii) The Nernst equation can be written as follows
Ecell = E cell – log
Ecell = Ecell + 0.059 Ag+
Here we observe that EAg+/Ag increases with increase in the concentration of
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Correct option: C
The assertion is valid, but the reasoning is erroneous because Arrhenius equation rate constants are accurate for both simple and complex molecules with suitable orientation during effective collision and sufficient kinetic energy to cause chemical change.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Ans: Correct Option: (i)
(i)At the equilibrium stage E cell = 0 and current stops flowing in the cell. Therefore, the assertion is correct, the reason is correct and the reason is the correct explanation for assertion. Therefore, this option is correct
(ii) Assertion is correct, the reason is correct and the reason is the correct explanation for assertion. Therefore, this option is incorrect
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
