Classification of Elements and Periodicity in Prop
Get insights from 123 questions on Classification of Elements and Periodicity in Prop, answered by students, alumni, and experts. You may also ask and answer any question you like about Classification of Elements and Periodicity in Prop
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The radius of Na+ ion is smaller than that of Na is due to the following reasons:-
(i) The effective nuclear charge of Na+
(ii) The disappearance of 3s orbital from its outermost shell electronic configuration.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Metallic character- Tendency to lose electrons.
Non-Metallic character- Tendency to accept electrons.
Across the period the metallic character decreases whereas non-metallic character increases across the period.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
(a) As the effective nuclear charge increases and shielding effect decreases across the periods thus the electronegativity increases on moving from left to right in the periodic table.
(b) As the number of shells increases down the group thus its ionisation enthalpy decreases down the group.
New answer posted
7 months agoContributor-Level 10
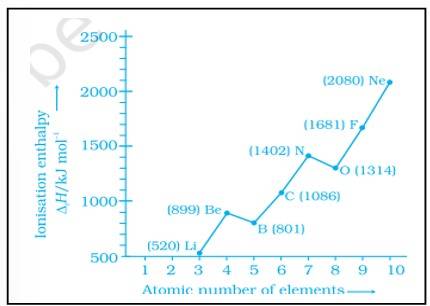
The deviation in the ionization enthalpy of some elements from the general trend can be explained by the points as given below:-
(i) The fully filled and half filled orbital provide extra stability due to the symmetry
(ii) The effective nuclear charge
(iii) The e- - e- repulsion which lead to instability
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
(i) S
The ionization enthalpy increases across the period and decreases down the group. N possesses a half filled 2p orbital which provides it extra stability due to symmetry.
(ii)P
The non-metallic character increases across the period
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Exothermic reaction- The reaction in which heat is released
For example- CaO + CO2 → CaCO3 + Heat
Endothermic reaction- The reaction in which heat is absorbed.
For example- 2NH3 + heat → N2 +3H2
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The electronic configuration of Na is [Ne]3s1 while that of Mg is [Ne]3s2 as Mg possesses a fully filled 3s orbital thus its first ionisation enthalpy is higher than that of Na.
After losing one electron Na obtained the electronic configuration of Ne while Mg acquired the electronic configuration of Na thus the second ionisation energy of Na is higher than that of Mg.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
In the p block, some elements are metallic some elements are non-metallic while some elements are metalloids in nature. The oxides of metals are basic in nature and that of oxides of nonmetals are acidic in nature.
Acidic oxide SO2 + H2O →H2SO3
Basic oxide Tl2O + 2HCl → 2TlCl +H2O
Amphoteric oxide
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Al2O3 +2 NaOH → 2NaAlO2 + H2O
Reaction with water
B3O2 +3H2O → 2H3BO3
P4O11 + 6H2O → 4H4PO3
Cl2 O7 + H2O → HClO4
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The anomalous behaviour of the first member of each group of representative elements i.e. of second period can be attributed to their small size, high charge/radius ratio, high electronegativity and absence of vacant d-orbitals to expand their oxidation state.The first member of each group of p-Block elements displays their greater ability to form multiple bond with itself, e.g. C=C, O=O, N=N and to other second periodic elements, e.g. C=O, C=N, N=N.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The N possesses a half filled p-orbital which provides it extra stability due to symmetry due to which its electron gain enthalpy is positive and its ionization enthalpy is larger than that of O.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers