Classification of Elements and Periodicity in Prop
Get insights from 123 questions on Classification of Elements and Periodicity in Prop, answered by students, alumni, and experts. You may also ask and answer any question you like about Classification of Elements and Periodicity in Prop
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The electronic configuration of Cr= 1s2 2s2 2p6 3s2 3p6 4s1 3d10
The electronic configuration of Cr after losing one electron= 1s2 2s2 2p6 3s2 3p6 3d10
The electronic configuration of F= 1s2 2s2 2p6 3s2 3p5
The electronic configuration of F after gaining one electron= 1s2 2s2 2p6 3s2 3p6
From the above electronic configuration, we can see that chromium will achieve stable electronic configuration after losing one 4s electron and fluorine will achieve stable electronic configuration after gaining one electron. So, the oxidation of chromium will be +1 and that of fluorine w
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Option (i)
As across the period the atomic radius decreases due to the increase of effective nuclear charge.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
The characteristics properties of p-block elements are as follows:-
(i) It contain metals, nonmetals and metalloids
(ii) Mostly involved in covalent bonding
(iii) Some elements show variable oxidation state
(iv) It possesses relatively higher ionization enthalpy compared to the s-block elements.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
(i) C has the highest ionization energy among the given elements as along the period ionization enthalpy increases whereas it decreases down the group.
(ii) Al is the most metallic element among the given elements because down the group metallic character increases
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
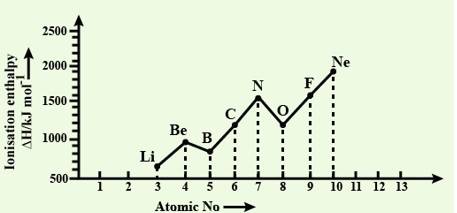
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Group | 1 |
Valency | 1 |
Outermost electronic configuration | 8s1 |
Formula of Oxide | M2O |
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Transition elements are named so because they form a bridge between s-block elements and p-block elements. Zn, Cd and Hg are among those elements that are dblock elements but they do not exhibit most of the properties of transition elements.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
As the atomic size of F is much smaller which leads to high e- - e - repulsion upon addition of electrons thus its electron gain enthalpy is less than that of Cl.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: As we move across the periodic table the ionization energy increases because of the increase in the effective nuclear charge and decrease of the shielding effect as more and more electrons get added in the same orbital.
Thus group 1 has lower ionization enthalpy compared to that of group 17 and also group 1 by losing one electron it will acquire the nearest noble gas electronic configuration which also contributes towards its lower ionization enthalpy.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
The following points make long form of periodic table better than Mendeleev's periodic table :-
(a) It is a periodic function of atomic number
(b) Elements are grouped as per there outermost electronic configuration
(c) Proper segregation of metals and non-metals
(d) More appropriate position of group VII.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers