Hydrogen
Get insights from 182 questions on Hydrogen, answered by students, alumni, and experts. You may also ask and answer any question you like about Hydrogen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
In both reactions H2O2 act as reducing agent
In reaction (A) HOCl – O.A
H2O2 – R.A
In reaction (B) I2 – O.A
H2O2 – R.A
New answer posted
5 months agoContributor-Level 10
A, B and C are correct statements. The H-H bond dissociation enthalpy is the highest for a single bond between two atoms of any element. Hydrogen does not reduce oxides of metals that are more active than iron.
New answer posted
5 months agoContributor-Level 9
H? O? can act as both an oxidizing & reducing agent in both acidic & basic medium. In the hydrogen economy, energy is stored & transmitted in the form of dihydrogen.
New answer posted
5 months agoContributor-Level 10
In sodium hydride (NaH), hydrogen has an oxidation state of -1. In this state, it can only act as a reducing agent.
New answer posted
5 months agoContributor-Level 10
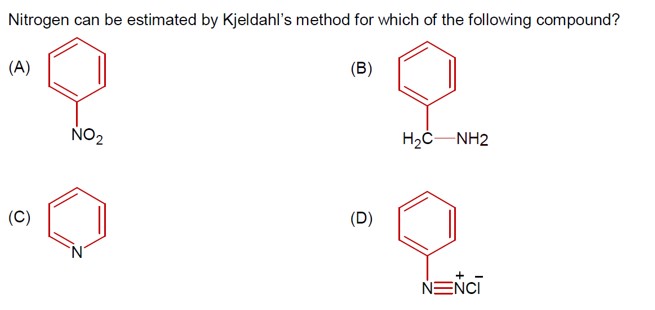
The Kjeldahl method is not applicable for nitrogen estimation in:
Compounds containing nitrogen in a nitro group.
Compounds containing an Azo group.
Pyridine.
New answer posted
5 months agoContributor-Level 10
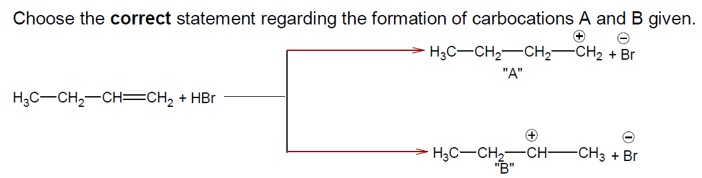
The addition of HBr to H? C−CH? −CH=CH? proceeds via carbocation formation.
Formation of a 1° Carbocation (A): H? C−CH?
Formation of a 2° Carbocation (B): H? C−CH? −C? H−CH?
The 2° carbocation (B) is more stable than the 1° carbocation (A). Therefore, the activation energy (Ea) for the formation of B is lower, and B is formed faster.
New answer posted
5 months agoContributor-Level 10
A diagonal relationship is observed in the periodic table between elements of period (2) and period (3).
Li and Mg
Be and Al
B and Si
Li and Na do not show a diagonal relationship.
New answer posted
5 months agoContributor-Level 10
Except for option (D), all are the important characteristics of D? O. D? O is useful for the isotopic leveling effect in reaction mechanisms. The dielectric constants of H? O and D? O are respectively 80 and 60.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers