Hydrogen
Get insights from 182 questions on Hydrogen, answered by students, alumni, and experts. You may also ask and answer any question you like about Hydrogen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
option (ii)
Its tendency to gain a single electron in its valence shell to attain stable electronic configuration.
Like halogens (with ns2np5 configuration belonging to the seventeenth group of the periodic table), it is short by one electron to the corresponding noble gas configuration, helium (ls2).
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
H2O (l)+NH3 (aq) → OH- (aq)+NH4+ (aq)
H2O (l)+H2S (aq) → H3O+ (aq)+HS- (aq)
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
2F2 (g)+2H2O → 4H+ (aq)+4F- (aq)+O2 (g)
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
In water hybridisation of oxygen is sp3. Angle should be109o (approx.) but due to LP - LP repulsion bond angle reduces to 104.5°.
New answer posted
8 months agoPhosphoric acid is preferred over sulphuric acid in preparing hydrogen peroxide from peroxides. Why?
Contributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Because H2SO4 can activate the decomposition of H2O2.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
With soap, hard water generates scum/precipitate. Soap containing sodium stearate (C117H35COONa) interacts with hard water to form calcium and magnesium stearate (Ca/Mg stearate). As a result, it is unsuitable for laundering. Because of the deposition of salts in the form of scale, it is also hazardous to boilers.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Hydrogen peroxide is stored in wax lined bottles because decomposes slowly on exposure to light.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Hydrogen peroxide cannot be concentrated by heating as it can cause bumps on heating. It can be extracted with water and concentrated to ~30% (by mass) by distillation under reduced pressure. It can be further concentrated to ~85% by careful distillation under low pressure. The remaining water can be frozen out to obtain pure H2O2.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Water has a significantly higher boiling point than hydrogen sulphide because of the presence of strong hydrogen bonds between water molecules, which are absent in hydrogen sulphide due to the lower electronegativity of sulfur compared to oxygen; this means more energy is required to separate water molecules, resulting in a higher boiling point.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
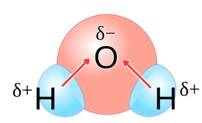
Water molecules are polar because in the gas phase water is a bent molecule with a bond angle of 104.5°, O-H bond length of 95.7 pm. Oxygen is more elsectronegative (EN = 3.5 ) than hydrogen (EN= 2.1 ) hence, O-H bond is polar . In the water molecule, two polar O-H nonds area present which are held together at an angle of 104.5 . Due to the resultant of these two dipoles, water molecule is polar and has an dipole moment of 1.84 Debye.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
