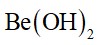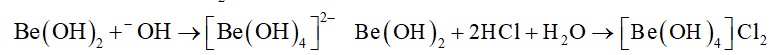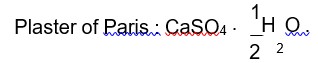Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Sodium carbonate is a white crystalline solid which exists as a decahydrate, 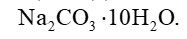
New answer posted
3 months agoNew answer posted
3 months agoContributor-Level 10
Amphoteric oxides are those which can react with both acid and base
SnO2, PbO2 and Al2O3 are amphoteric oxide
SiO2, P2O5, CO2 are acidic oxides
CO, NO and N2O are neutral oxides
New answer posted
3 months agoContributor-Level 10
Suspension of slaked lime in water is known as milk of lime
Crude sodium chloride obtained by crystallisation of brine solution contains CaSO4, Na2SO4 and MgCl2
Beryllium salts are easily hydrolysed
Gypsum : CaSO4 * 2H2O
New answer posted
3 months agoContributor-Level 10
Kindly go through the solution
Since VReal > Videal
So, Z = VReal > 1. Videal
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers