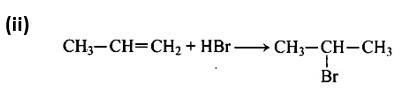Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Because halogen atoms are ortho and para directing, rather than ring deactivators, further electrophilic substitution occurs at ortho and para locations.
Correct Answer: Option (v)
New answer posted
4 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
The nitro group on the ortho and para positions of haloarenes acts as an electron-withdrawing group, making it more reactive to nucleophilic substitution reactions due to the electron deficit.
Correct Answer: Option (i)
New answer posted
4 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Because tertiary butyl bromide interacts with NaI in dry ether to create 2, 3, 3-tetramethylbutane, it undergoes the Wurtz reaction to yield 2, 3, 3-tetramethylbutane.
Correct Answer: Option (i)
New answer posted
4 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Haloalkanes react with AgCN to produce alkyl isocyanides as the primary product, whereas KCN produces alkyl cyanides.
Correct Answer: Option (iv)
New answer posted
4 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Because of the size of the halogen atom, the boiling temperatures of alkyl halides drop in the specified sequence. With the greatest atomic number, iodide has the most electrons, resulting in increased Van Der Waals forces and a higher boiling point.
Correct Answer: Option (v)
New answer posted
4 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Thionyl chloride is favoured over phosphorus chlorides because the by-products produced, in addition to the alkyl halides, include SO2 and HCl, which are gaseous and so may escape the reaction, leaving just pure halides.
Correct Answer: Option (ii)
New answer posted
4 months agoContributor-Level 10
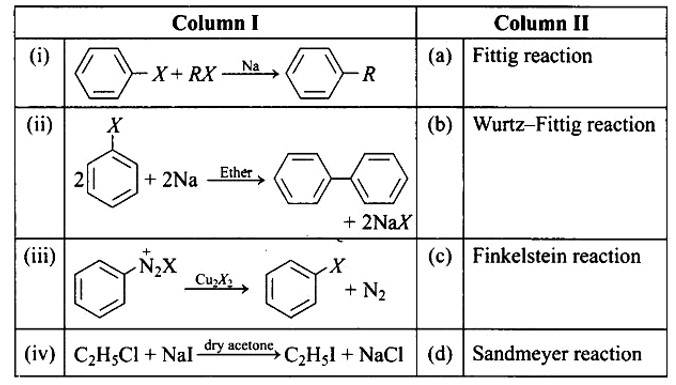
This is a matching answer type question as classified in NCERT Exemplar
In the presence of Na metal in dry ether, an alkyl halide and an aryl halide combine to create alkyl arene in the Wurtz-Fittig reaction.
The Fittig reaction, which involves a coupling reaction between two aryl halides in dry ether in the presence of Na metal, yielding diaryl compounds, is the second reaction.
The Sandmeyer reaction produces aryl halides from primary amines by converting them to diazonium salts and treating them with cuprous bromide.
The Finkelstein reaction, which produces alkyl iodides by reacting alkyl chlorides or bromides with NaI in dry acetone, i
New answer posted
4 months agoContributor-Level 10
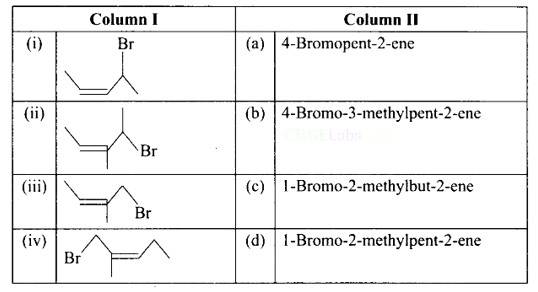
This is a matching answer type question as classified in NCERT Exemplar
(i) A: 4-bromopent-2-ene.
(ii) B: 4-bromo-3-methylpent-2-ene.
(iii) C: 1-bromo-2-methylbut-2-ene.
(iv) D: 1 -bromo-2-methylpent-2-ene.
Correct Answer: (i) → (a) (ii) → (c) (iii) → (b) (iv) → (d)
New answer posted
4 months agoContributor-Level 10
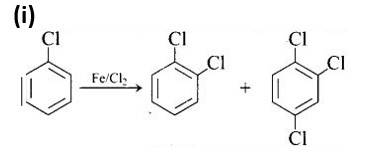
This is a matching answer type question as classified in NCERT Exemplar
The first reaction is an electrophilic substitution process in which Cl+ from the Cl2 and FeCl3 combination attacks and substitutes the benzene ring.
Following Markownikoff's rule, the second reaction is an electrophilic addition reaction in which HBr is added across the double bond.
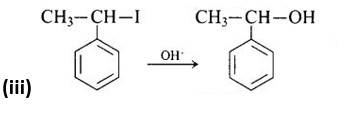
The third reaction is a nucleophilic substitution process that uses the SN1 mechanism to replace the -I group with -OH.
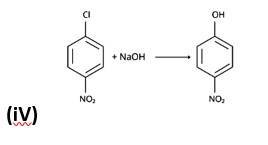
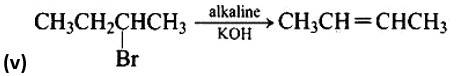
The nucleophilic aromatic substitution process, in which the -Cl group is replaced by -OH, is the fourth reaction.The fifth reaction is a dehydrohalogenation reacti
New answer posted
4 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
Because it includes a halogen atom bound to an sp3 hybridised carbon atom, which is subsequently linked to additional alkyl groups, the first molecule is an alkyl halide.
An allyl halide is a compound in which the halogen atom is linked to a carbon atom that is bound to an sp3 hybridised carbon atom that is attached to a carbon-carbon double bond.
The third molecule is an aryl halide, which is a chemical in which the halogen atom is sp2 hybridised with an aromatic ring carbon atom.
In a carbon-carbon double bond, the halogen atom is linked to a sp2 hybridised carbon at
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers