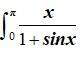Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Complexes with more number of ions show more conductivity.
[Co (NH3)3Cl3], [Co (NH3)4Cl2] < [Cr (NH3)5Cl]Cl2
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: The octahedral and tetrahedral splitting up of d orbitals is different.
Δt= ( )Δ0
Δt < 0
Δt= CFSE in tetrahedral field
Δ0= CFSE in octahedral field
Comparing CFSE with energy=
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: When light wavelengths from a specific part of the spectrum are absorbed by a substance, the result is a complimentary colour. When a complex absorbs a wavelength of light, it reflects a complementary colour. If a violent colour is absorbed, for example, yellow is conveyed. The CFSE value, often known as the colour definer for any complex, comes next. To determine the wavelength value in order to determine which colour absorbs the most energy.
Δe=
As λ has shorter wavelength.
Low spin complexes absorb shorter wavelengths, while high spin complexes absorb long
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: (i) 'A' is [Co (NH3)5SO4]Cl
'B' is [Co (NH3)5Cl]SO4
(ii) Type of isomerism is ionisation isomerism
(iii) IUPAC name of isomer 'A' is pentaaminesulphatocobalt (III)chloride
IUPAC name of isomer 'B' is pentaaminechlorocobalt (III)sulphate
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans:
[Mn (CN)6]3−
Electronic configuration is Mn3+= [Ar]3 d4 hence box electronic structure
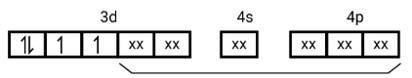
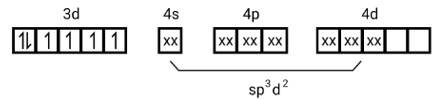
(i) Type of hybridisation d2sp3
(ii) Inner orbital complex
(iii) paramagnetic, due to presence of three unpaired electrons.
(iv) Spin only magnetic moment is calculated using the formula : n=2 in this case, we get spin only magnetic moment in BMas = = = 2.87BM
[Co (NH3)6]3+
Electronic configuration of Co3+= [Ar]3 d6
(i) Hyb As shown in the above box electronic structure the type of hybridisation is . d2sp3
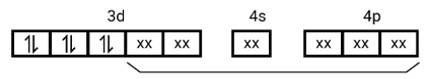
(ii) Inner orbital complex
(iii) Diamagnet
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
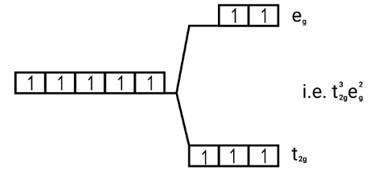
Ans: (i) Electronic cnfiguration: Co3+ =[Ar]3d6
Energy level diagram:
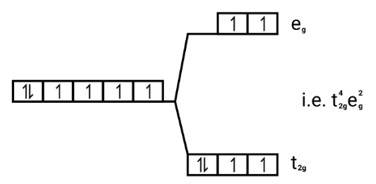
Magnetic moment:
Number of unpaired electrons (n)=4
Magnetic moment = μ= =
= = 4.9 BM
[Co(H2O)6]2+
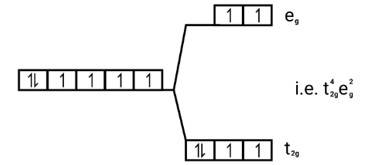
Electronic cnfiguration: Co2+=[Ar]3 d7
Energy level diagram:
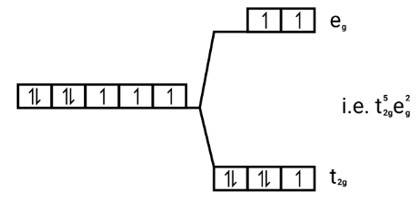
Magnetic moment: Since ,number of unpaired electrons (n)=3, therefore magnetic moment = = = 3.87BM
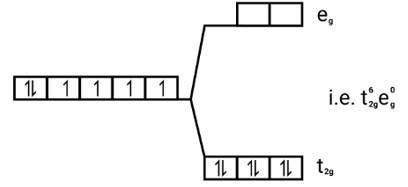
[Co(CN)6]3−
Electronic configuration: [Ar]Co3+=3 d6
Energy level diagram:
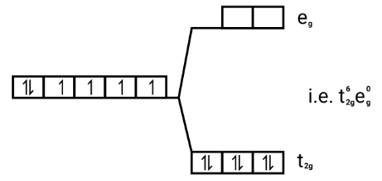
Ans: [FeF6]3−
Electronic configuration: Fe3+=[Ar]3 d5
Energy level d
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
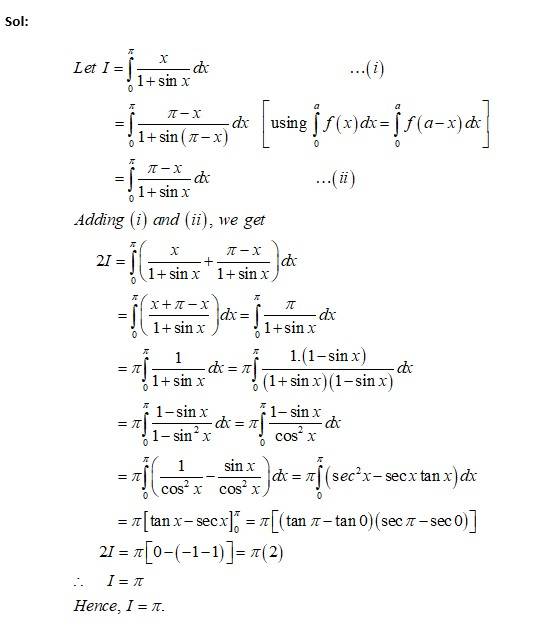
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
4 months agoContributor-Level 10
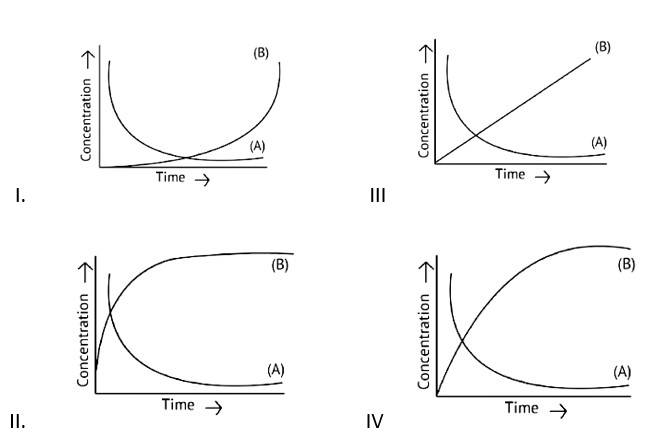
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
Ans: Correct option B
If A→B then the concentration of both reactants and the products vary exponentially with time. But, in option B graph the reactant concentration decreases exponentially and the product concentration increases.
New answer posted
4 months agoContributor-Level 10
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
Ans: Correct option C
Let's start with what a pseudo-first-order response is.
Although the pseudo-first-order reaction looks to be an order, it belongs to another order. It's a second-order reaction because it involves two reactants.
Let's have a look at a reaction.
CH3Br + OH→CH3OH + Br-
So, the rate law for the reaction is
Rate = k [OH] [CH3Br]
Rate = k [OH- ] [CH3Br] = k (constant) [CH3Br] = K' [CH3Br]
Only the concentration of CH3Br will change during the reaction, and the rate will be determined by the reaction's modifications.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers