Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Because it is a tertiary alkyl halide, the described reaction is a nucleophilic substitution that follows the SN1 mechanism. It's a two-step process. The first step is the gradual breaking of a polarised C-Br bond, which results in the formation of a carbocation and a bromide ion. To complete the substitution, the stable intermediate carbocation is attacked by the nucleophile OH−. (i) and (iv) are the right statements.
Correct Answer: option (A) and (iv)
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Because the carbon atom is transiently linked to five atoms, the intermediate (iii) is less stable than reactant (ii), which is coupled to four groups.
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
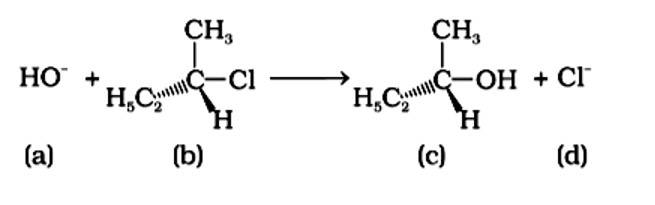
An SN2 reaction, also known as a nucleophilic substitution, is the reaction in question. The entering nucleophile causes the groups surrounding the carbon atom to migrate in the opposite direction of the nucleophile, causing the configuration of alkyl halides to invert. As a result, (b) and (d) will be in the opposite order. This reaction is also SN2 since there is just one phase in which OH- is added and Cl- is removed at the same time. (i) and (ii) are the right answers.
Correct Answer: Option (A) and (B)
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
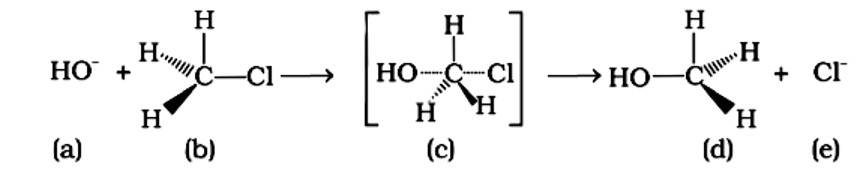
An SN2 nucleophilic substitution reaction occurs when CH3Cl interacts with the hydroxide ion to produce CH3OH. Both OH - and Cl - are nucleophiles with an excess of electrons in this reaction. Because the binding of OH - and the leaving of Cl - occurs simultaneously in the transition state (c), the carbon atom is linked to just three hydrogens. As a result, the carbon atom becomes sp2 hybridised. Aand (C) are the right statements.
Correct Answer: Option (A) and (C)
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
The Correct Answer is Option (iv).
If the halogen atom in all of the supplied alkyl halides is the same kind, the boiling point of a compound rises as the number of alkyl groups added increases. As the quantity and size of molecules and electrons grow, so do their attractions. As a result, the greater the boiling point, the larger the molecular weight of the alkyl halide. The boiling points of the following compounds are listed in ascending order: 1-Bromoethane 1-Bromopropane 1-Bromobutane Bromobenzene The right answer is (iv).
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
The Correct Answer is Option (i).
The intermolecular force of attraction will grow as the surface area increases. As a result, the boiling point rises as well. The boiling point of a comparable alkyl halide rises with increasing molecular mass. Iodine has the greatest atomic mass. As a result, 1-iodobutane has the greatest boiling point.
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
The Correct Answer is Option (iii).
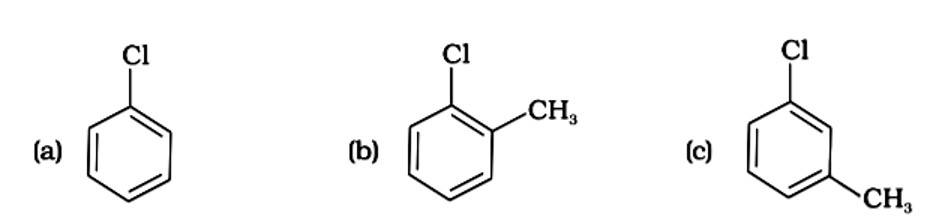
The presence of electron releasing groups increases the reactivity of aryl halides; the fewer the electron releasing groups, the slower the rate of nucleophilic substitution. As a result, an increase in methyl groups decreases reactivity. The sequence of reactivity is (a) > (b) > (c). The right answer is (iii).
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
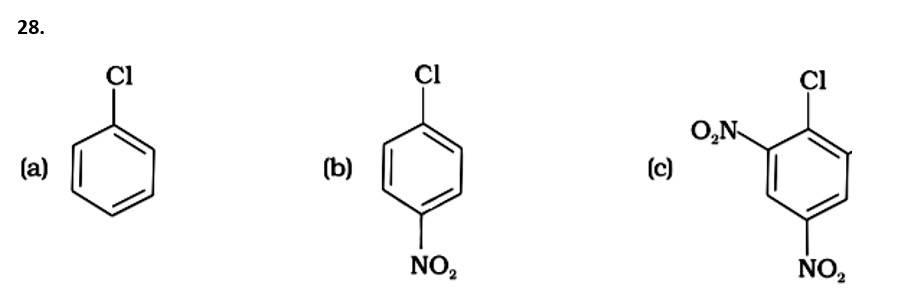
The Correct Answer is option (iv).
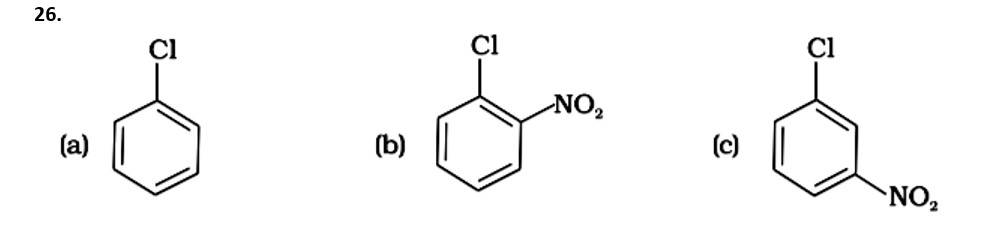
( - NO2) is an electron-withdrawing group that induces nucleophilic substitution when it is in the ortho and para positions. When this identical electron-drawing group is in the meta position, its impact is much reduced. The reactivity of aryl halides rises as the number of electron-withdrawing groups increases. As a result, the greater the number of electron-drawing groups, the greater the rate of nucleophilic substitution.
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
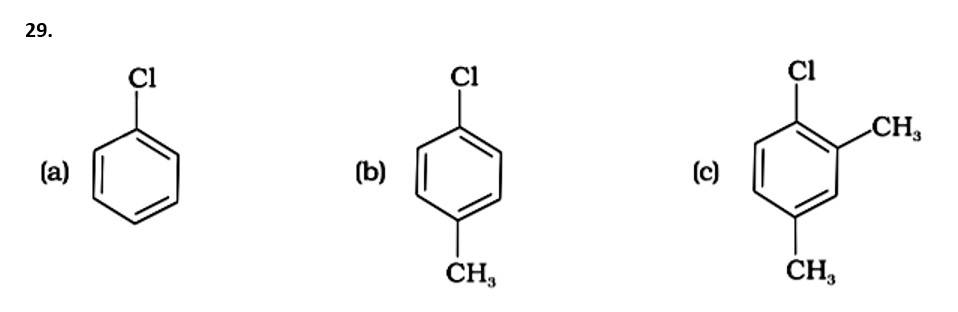
The Correct Answer is Option (iv).
Because the -CH3 group is an electron releasing group, it reduces the reactivity of aryl halides in the ortho and para locations. As a result, aryl halides without electron releasing groups are more reactive. As a result, the order of reactivity is (a) > (c) > (d) (b). The right answer is (iv).
New answer posted
4 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
The Correct Answer is Option (iii).
Due to the resonance stabilisation of the benzene ring, the reactivity of aryl halides to nucleophilic substitution is exceedingly low. Because of resonance, the -Cl bond gains a partial double bond. The presence of an electron withdrawing -NO2 group at ortho or para positions on the ring enhances the reactivity of aryl halides. The presence of -NO2 near the C-Cl makes the molecule more reactive. The order of reactivity should be (b) > (c) > (a). The right option is (iii).
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers