Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
5 months agoNew answer posted
6 months agoContributor-Level 10
4.39 Given, k2 = 4k1, T1 = 293K and T2 = 313K
We know that from the Arrhenius equation, we obtain
On solving, we get,
Ea = 58263.33 J mol-1 or 58.26 kJ mol-1
New answer posted
6 months agoContributor-Level 10
4.38 We know, time t = (2.303/k) * log ( [R]0/ [R])
Where, k- rate constant
[R]0-Initial concentration
[R]-Concentration at time 't'
At 298K, If 10% is completed, then 90% is remaining. t = (2.303/k) * log ( [R]0/0.9 [R]0)
t = (2.303/k) * log (1/0.9) t = 0.1054 / k
At temperature 308K, 25% is completed, 75% is remaining t' = (2.303/k') * log ( [R]0/0.75 [R]0)
t' = (2.303/k') * log (1/0.75) t' = 2.2877 / k'
But, t = t'
0.1054 / k = 2.2877 / k' / k = 2.7296
From Arrhenius equation, we obtain log k2/k1 = (Ea / 2.303 R) * (T2 - T1) / T1T2
Substituting the values,
Ea = 76640.09 J mol-1 or 76.64 kJ mol-1 We know, log k = log A –Ea/RT
Log k =
New answer posted
6 months agoContributor-Level 10
4.37 From Arrhenius equation, we obtain
Also, k1 = 4.5 * 103 s -1
T1 = 273 + 10 = 283 K
k2 = 1.5 * 104 s -1
Ea = 60 kJ mol -1 = 6.0 * 104 J mol -1
Then,
→ 0.5229 = 3133.627 * (T2-283)/ (283 * T2)
→ 0.0472T2 = T2-283 T2 = 297K or T2 = 240 C
New answer posted
6 months agoContributor-Level 10
4.36 We know, The Arrhenius equation is given by k = Ae-Ea/RT Taking natural log on both sides,
Ln k = ln A- (Ea/RT)
Thus, log k = log A - (Ea/2.303RT). eqn 1
The given equation is log k = 14.34 – 1.25 * 104K/T. eqn 2
Comparing 2 equations, Ea/2.303R = 1.25 * 104K
Ea = 1.25 * 104K * 2.303 * 8.314
Ea = 239339.3 J mol-1 (approximately) Ea = 239.34 kJ mol-1
Also, when t1/2 = 256 minutes,
k = 0.693 / t1/2
= 0.693 / 256
= 2.707 * 10-3 min-1 k = 4.51 * 10-5s–1
Substitute k = 4.51 * 10-5s–1 in eqn 2,
log 4.51 * 10-5 s–1 = 14.34 – 1.25 * 104K/T
log (0.654-5) = 14.34– 1.25 * 104K/T = 1.25 * 104/ [ 14.34- log (0.654-5)] T = 668.9K or T =
New answer posted
6 months agoContributor-Level 10
4.35 The given equation is
k = (4.5 x 1011 s-1) e-28000K/T (i)
Comparing, Arrhenius equation
k = Ae -E/RT (ii)
We get, Ea / RT = 28000K / T
⇒Ea = R x 28000K
= 8.314 J K-1mol-1 * 28000 K
= 232792 J mol–1 or 232.792 kJ mol–1
New answer posted
6 months agoContributor-Level 10
4.34 t1/2 = 3.00 hours
We know, t1/2 = 0.693/k
? k = 0.693/3 k = 0.231 hrs-1
We know, time
Where, k- rate constant
[R]° -Initial concentration
[R]-Concentration at time 't'
Thus, substituting the values,
log ( [R]0/ [R]) = 0.8
log ( [R]/ [R]0) = -0.8
[R]/ [R]0 = 0.158
Hence, 0.158 fraction of sucrose remains.
New answer posted
6 months agoContributor-Level 10
4.33 Given,
k = 2.0 * 10–2s-1
time t = 100s
Concentration [A0] = 1.0 mol L-1
We know,
On substituting the values, Log (1/ [A]) = 2.303/2
Log [A] = -2.303/2 [A] = 0.135 mol L–1
New answer posted
6 months agoContributor-Level 10
4.32 Given,
k = 2.418 * 10-5 s-1
T = 546 K
Ea = 179.9 kJ mol-1 = 179.9 * 103J mol-1
The Arrhenius equation is given by k = Ae-Ea/RT Taking natural log on both sides,
Ln k = ln A- (Ea/RT) Substituting the values,
ln (2.418 * 10-5 ) = ln A-179.9/ (8.314 * 546)
ln A = 12.5917
A = 3.9 * 1012 s-1 (approximately)
New answer posted
6 months agoContributor-Level 10
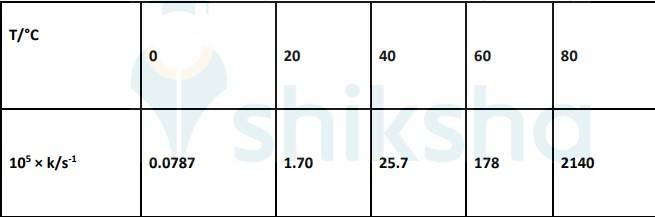
4.31 To convert the temperature in °C to °K we add 273 K.
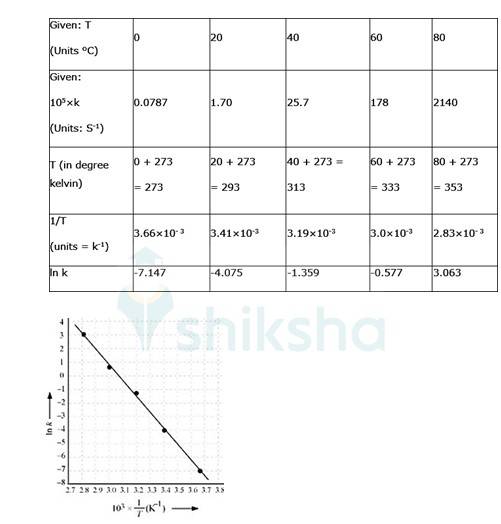
The graph is given as:
The Arrhenius equation is given by k = Ae-Ea/RT
Where, k- Rate constant
A- Constant
Ea-Activation Energy
R- Gas constant
T-Temperature
Taking natural log on both sides,
ln k = ln A- (Ea/RT). equation 1
By plotting a graph, ln K Vs 1/T, we get y-intercept as ln A and Slope is –Ea/R.
Slope = (y2-y1)/ (x2-x1)
By substituting the values, slope = -12.301
? –Ea/R = -12.301
But, R = 8.314 JK-1mol-1
? aE= 8.314 JK-1mol-1 * 12.301 K
? aE= 102.27 kJ mol-1
Substituting the values in equation 1 for data at T = 273K
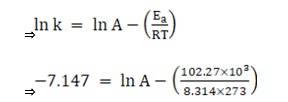
(? At T = 273K, ln k =-7.147)
On solving, we get ln A = 37.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers