Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
4.30 When t = 0, the total partial pressure is P0 = 0.5 atm
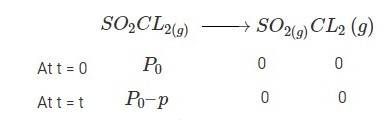
When time t = t, the total partial pressure is Pt = P0 + p
P0-p = Pt-2p, but by the above equation, we know p = Pt-P0
Hence, P0-p = Pt-2 (Pt-P0)
Thus, P0-p = 2P0 – Pt
We know that time
t= 2.303/K log R0 / R
Where, k- rate constant
[R]° -Initial concentration of reactant [R]-Concentration of reactant at time 't'
Here concentration can be replaced by the corresponding partial pressures.
Hence, the equation becomes,
t= 2.303/K log P0 / P0 - P
t= 2.303/K log P0 / 2P0 - Pt
? equation 1
At time t = 100 s, Pt = 0.6 atm and P0 = 0.5 atm,
Substituting in equation 1,
100 = 2.303/k log 0.5 /
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
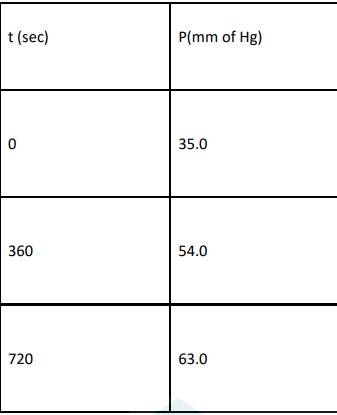
4.29 When t = 0, the total partial pressure is P0 = 35.0 mm of Hg
When time t = t, the total partial pressure is Pt = P0 + p
P0-p = Pt-2p, but by the above equation, we know p = Pt-P0 Hence, P0-p = Pt-2 ( Pt-P0)
Thus, P0-p = 2P0 – Pt
We know that time
t= 2.303/K log R0 / R
Where, k- rate constant
[R]° -Initial concentration of reactant
[R]-Concentration of reactant at time 't'
Here concentration can be replaced by the corresponding partial pressures.
Hence, the equation becomes,
t= 2.303/K log P0 / P0 - P
t= 2.303/K log P0 / 2P0 - Pt
? equation 1
At time t = 360 s, Pt = 54 mm of Hg and P0 = 30 mm of Hg, Substituting in equation 1,
360 = 2
New answer posted
6 months agoContributor-Level 10
7.70
As Bond dissociation energy generally decreases on moving down the group as the atomic size of the element increases. However, among halogens, the bond dissociation energy of F2 is lower than that of Cl2 and F2 due to the small atomic size of
Thus increasing order for bond dissociation energy among halogens is as follows:I22
As Bond dissociation energy of H-X molecules where X is the halogen decreases with increase in the atomic HI is the strongest acid as it loses H atom easily due to weak bonding between H and I.
So Increasing acid strength is as follows: HF
Basic strength decreases as we move from Nitrogen to Bismuth down the group
New answer posted
6 months agoContributor-Level 10
7.69
(i) XeO3 can be produced by hydrolysis of XeF4 and XeF6 under controlled pH of the medium in which reaction is taking place as shown below:
6XeF4 + 12H2O → 4Xe + 2XeO3 + 24HF + 3O2
XeF6 + 3H2O → XeO3 + 6HF
(ii) XeOF4 can be obtained on partial hydrolysis of XeF6 as shown below:
XeF6 + H2O → XeOF4 + 2HF
New answer posted
6 months agoContributor-Level 10
7.68
ClO- isisoelectronic to ClF as both the compounds contain 26 electrons in all. ClO- : 17+8+1 = 26
ClF : 17+9 = 26
Yes, ClF Molecule is a Lewis base as it accepts electrons from F to form ClF3.
New answer posted
6 months agoContributor-Level 10
7.33
Xe and F2 combine under different conditions to produce XeF2, XeF4, XeF6 as follows:
Ratio | Temperature & Pressure Condition | Reaction |
Excess | at {673K,1bar} | Xe (g) + F2 (g) → XeF2 (s) |
1:5 ratio | at {873K,7bar} | Xe (g) + 2F2 (g) → XeF4 (s) |
1:20 ratio | at {573K,60-70bar} | Xe (g) + 3F2 (g) → XeF6 (s) |
New answer posted
6 months agoContributor-Level 10
7.66
4NaCl + MnO2 + 4H2SO4 MnCl2 + 4NaHSO4 + 2H2O + Cl2
Manganese (IV) oxide reacts with sodium chloride and sulfuric acid to produce manganese (II) chloride, chlorine, sodium bisulfate and water.
This reaction takes place at a temperature near 100°C.
Cl2 + NaI 2NaCl + I2
Chlorine reacts with sodium iodide to produce sodium chloride and iodine. Chlorine - diluted solution.
Sodium iodide - cold solution.
New answer posted
6 months agoContributor-Level 10
7.64
Neil Bartlett first performed an experiment in which reaction between oxygen and PtF6 was carried out which lead to the formation of a red coloured compound O2 + [PtF6 ]-.
He observed that the first ionization energy of Oxygen and Xenon is almost same (~1170 kJ/mol). So, he tried to react Xe and PtF6 in which he was successful to obtain a red coloured compound Xe+ [PtF6]-.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers