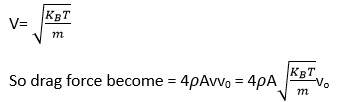physics ncert solutions class 11th
Get insights from 951 questions on physics ncert solutions class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about physics ncert solutions class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Mean free path l=1/
So n= number of molecules /volume
d = diameter of the molecule
l
d1=1Ao, d2=2Ao
l
l1:l2=4:1
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Oxygen gas having 5 degrees of freedom
Energy per mole of the gas =5/2RT
For 2 moles of the gas total internal energy =2 5/2RT=5RT
Neon is a monoatomic gas having 3 degrees of freedom
Energy per mole =3/2RT
We have 4 moles of Ne
Energy = 4 3/2RT=6RT
Total energy =5RT+6RT=11RT
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
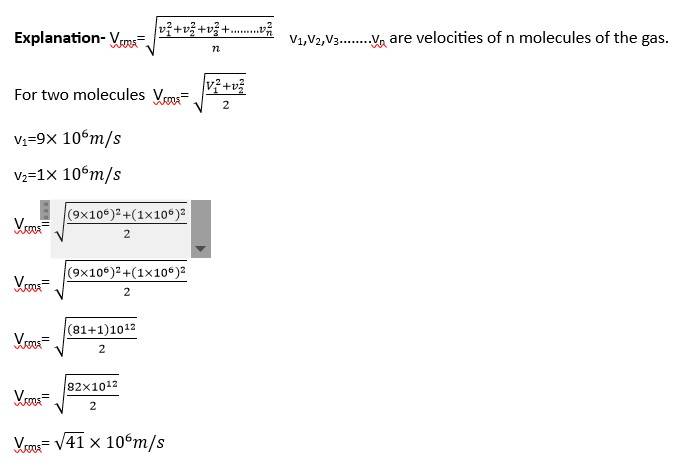
Vrms=
Vrms=
T1=27oC= 27+273=300K
T2=127oC= 127+273= 400K
Vrms2=
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
V
V/T = constant
T1=273+27=300K
T2= 273+327= 600K
V1= 100cc
V2=V1 (600/300)
V2=2V1
V2= 2 (100)=200cc
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
molar mass = mass of avogadro's number of atoms= 6.023 atoms.
197 g of gold contains =6.023
1g of gold contain= atoms
39.4 g of gold atoms = atoms
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Consider the diagram
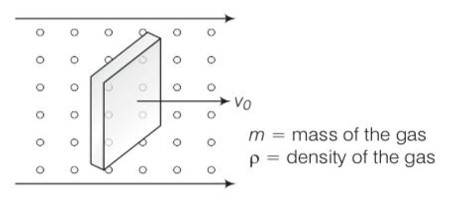
let n =number of molecules per unit volume
Vrms= rms speed of gas molecule
When block is moving with speed vo, relative speed of molecules w.r.t front face =v+vo
Coming head on, momentum transferred to block per collision =2m (v+vo)
Number of collisions in time = (v+vo)n A where A is the area of cross section.
So momentum transferred in time =m (v+vo)2nA this is from front surface
Similarly momentum transferred in time = m (v-vo)2nA ) this is from back surface
Drag force = mnA (v+vo)2- (v-vo)2)
= mnA (4wo)=4mnAvvo
= 4 vvo
So =mn/V=M/
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Given volume V = 1m3
area = 0.01mm2
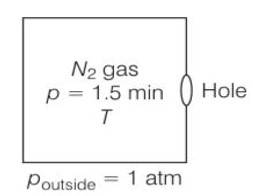
= 8.01 m2= m2
Temperature both inside and outside are equal
So initial pressure inside the box = 1.50atm
Final pressure inside the box= 0.1atm
Assuming Vx= speed of nitrogen molecule in x direction
ni = number of molecules per unit volume in a time interval of
Let area of the wall, number of particles colliding in time
= i (vx )A , here we use ½ because particle moves both in positive and negative direction.
Vx2+ Vy2+ Vz2= Vrms2
Vx2= Vrms2/3 if all three velocities are equal.
½ mvrms2= 3/2KBT/m


New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Time require to avoid the collision T= l/v where l = mean free path =1/
Where n = N/V
n=number of aeroplanes/volume
= -3
T=
T= =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers