Physics Ncert Solutions Class 12th
Get insights from 1.2k questions on Physics Ncert Solutions Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics Ncert Solutions Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- On removing one electron from He4 and He3, the energy levels, as worked out on the basis of Bohr model will be very close as both the nuclei are very heavy as compared to electron mass.Also after removing one electron from He4 and He3 atoms contain one electron and are hydrogen like atoms.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- the reason behind this is the binding energy or we can say the mass defect.
BE= mass defect (931MeV ). So whatever energy is liberated in fission and fusion changes the mass slighthly. That why there is a defect in mass
New answer posted
8 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- when r
Let
F=
Where = (
Let p=
Electrostatic force is balanced by centripetal force
Mv2/r or v2=
New answer posted
8 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- mp= 10-6
Mpc2=10-6
= 0.8
Wavelength associated with both of them is same
U(r)=-
Mvr=h
V=h/mr
Mv2/r=( ) ( )
13.6
New answer posted
8 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- the energy of nth state En=-Z2R where R is constant and Z=24
The energy release ina transition from 2 to 1
The energy required to eject a n=4 electron is E4= Z2R1/16
So kinetic energy of auger electron is, KE= Z2R (3/4-1/16)=1/16Z2R
=
New answer posted
8 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- in this type of situation centripetal force is balanced by electrostatic force
So mv2/r= -ke2/r2
According to bohr postuates
Mvr = h when n=1
On solving n
So after solving r= 0.51A0
So potential energy = -27.2eV , KE= mv2/2
=
But if R
If R>>r the electron moves inside the sphere with radius r'
Charge inside r'4=e
So r' =
So r'4= 0.51A0R3
= 510(A0)4
New answer posted
8 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- total energy of electron
E=
The frequency hv=
=
= =
=
When me<
after solving we get 2.714
New answer posted
8 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- as we know total energy in stationary orbit is
En=- where sign have usual meaning.
According to bohr third postulate h
-
Where is the reduced mass
Reduced mass for H=H=;me(1-me/M)
D= D; me(1-me/2M)
=me(1-me/2M)(1+me/2M)
If for hydrogen deuterium, the wavelength
= (1+ )-1= (1- )
so lines emitted are 1217.7A0,1027.7A0,974.04A0
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a, d) For house hold supplies, AC currents are used which are having zero average value over a cycle.
The line is having some resistance so power factor cos φ = R/Z≠0
so, φ not equal to π /2 ⇒ φ < /2
i.e., phase lies between 0 and π /2.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c, d) When the AC voltage is applied to the capacitor, the plate connected to the positive terminal will be at higher potential and the plate connected to the negative terminal will be at lower potential.
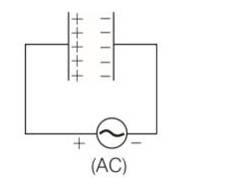
The plate with positive charge will be at higher potential and the plate with negative charge will be at lower potential. So, we can say that the charge is in phase with the applied voltage.
P= ErmsIrmscos
=90
So power, P = 0
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
