Redox Reactions
Get insights from 164 questions on Redox Reactions, answered by students, alumni, and experts. You may also ask and answer any question you like about Redox Reactions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
The reaction is: FeCl? + 3H? C? O? + 3KOH → K? [Fe (C? O? )? ] (A) + 3HCl + 3H? O
In the complex [Fe (C? O? )? ]³? , the oxalate ion (C? O? )²? is a bidentate ligand.
There are three bidentate ligands, so the coordination number, or secondary valency, of Fe is 3 * 2 = 6.
New answer posted
4 months agoContributor-Level 10
Cr? O? ²? + Fe²? - (H? )-> Cr³? + Fe³?
(n=6) (n=1)
Meq Cr? O? ²? = Meq Fe²?
20 * 0.03 * 6 = 15 * M * 1
M = (20 * 0.03 * 6) / 15 = 0.24 = 24 * 10? ²
Ans = 24
New answer posted
4 months agoContributor-Level 9
eq of H? O? = eq of KMnO?
(0.316 / 158) x 5 = 0.01
(0.01 x 17 / 0.2) x 100 = 85%
New answer posted
4 months agoContributor-Level 10
(i) 2Fe²? + H? O? → 2Fe³? + 2OH?
(ii) 2MnO? + 5H? O? + 6H? → 2Mn²? + 5O? + 8H? O
So sum of (x + y + x¹ + y¹ + z¹) = 2 + 2 + 2 + 5 + 8 = 19
New answer posted
4 months agoContributor-Level 9
2 Pb (NO? )? → 2PbO + 2NO? + O?
2NO? → N? O? colourless
N? O? + 2NO → 2 N? O?
Oxidation state in N? O? is +3
New answer posted
4 months agoContributor-Level 10
Toilet cleaner is acidic (HCl). Neutralize with weak base NaHCO?
New answer posted
4 months agoContributor-Level 10
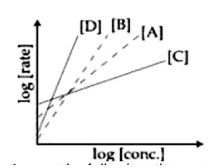
rate=k [Conc]? log [rate]=logk+nlog [Conc]. 'n' is slope. Higher slope, higher order.
New answer posted
4 months agoContributor-Level 10
50.00
K? Cr? O? + FeC? O? → Fe³? + CO? + Cr³? + K? + H? O
n_f=6 n_f=3
Now apply equivalent concept
0.02 * 6 * V = (0.288 / (144/3) * 10³
Normality
V = (0.288 * 10³)/ (48 * 6 * 0.02) = 50.00 mL
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers