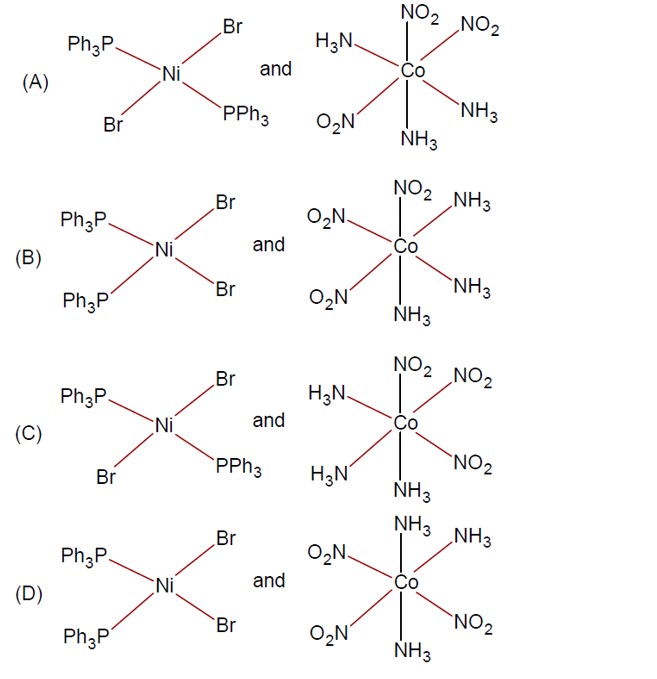
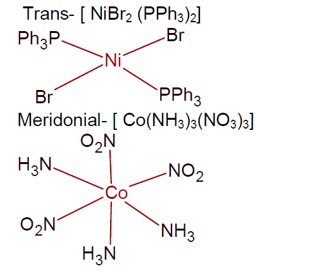
Solid State
Get insights from 127 questions on Solid State, answered by students, alumni, and experts. You may also ask and answer any question you like about Solid State
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Since, atom B forms CCP structure. Therefore, there will be 4-B atoms.
Now, atom A occupies 1/3 of tetrahedral voids.
Hence, number of A atoms = 1/3 * 8 = 8/3
The correct formula of the compound = A? /? B?
= A? B?
x + y = 2 + 3 = 5
New answer posted
5 months agoContributor-Level 10
n (O? ) = 4/32 = 1/8 mol
n (H? ) = 2/2 = 1 mol
n (Total) = n (O? ) + n (H? ) = 1/8 + 1 = 9/8 mol
PV = nRT
P (Total) * 1 = (9/8) * 0.082 * 273
P (Total) = 25.18 atm
New answer posted
5 months agoContributor-Level 10
Λ°m (NaCl) = 126.45Scm² mol? ¹
Λ°m (HCl) = 426.16Scm² mol? ¹
Λ°m (CH? COONa) = 91Scm² mol? ¹
Λ°m (CH? COOH) =Λ°m (CH? COONa) +Λ°m (HCl)−Λ°m (NaCl)
= 91 + 426.16 – 126.45
= 391.72Scm² mol? ¹
New answer posted
5 months agoContributor-Level 10
Both statements are correct independently but the reason is not the correct reason of the assertion.
New answer posted
5 months agoContributor-Level 9
Edge length in bcc, a? = 27 Å
Let, Edge length in fcc be a? Å
Now, the same element crystallises in bcc as well as fcc.
For bcc: 4r = √3 a? ⇒ r = (√3 / 4) a?
For fcc: 4r = √2 a? ⇒ r = a? / (2√2)
So, (√3 / 4) a? = a? / (2√2)
(√3 / 4) * 27 = a? / (2√2)
a? = 33.13 Å
The nearest integer is 33.
New answer posted
5 months agoContributor-Level 10
First, the number of unit cells in the given mass is determined. In an HCP structure, there are 6 atoms and 18 total voids (6 octahedral + 12 tetrahedral) per unit cell. Multiplying the number of unit cells by 18 gives the total number of voids. The result is14.9 x 10²¹, which is rounded.
New answer posted
5 months agoContributor-Level 9
Covalent solids have high melting point due to strong covalent bonds, also they are insulator in both solid as well as in molten state.
New answer posted
5 months agoContributor-Level 9
For an acidic buffer solution, pH = pKa + log ( [Base]/ [Acid]).
Given pH = 5.74 and pKa = 4.74.
5.74 = 4.74 + log ( [Base]/1).
1 = log ( [Base]).
[Base] = 10M.
New answer posted
5 months agoContributor-Level 10
For the dissociation of K? [Fe (CN)? ]? 4K? + [Fe (CN)? ]? , the number of ions produced (n) is 5.
The degree of dissociation (α) is related to the van't Hoff factor (i) by α = (i-1)/ (n-1).
Given α = 0.4: 0.4 = (i - 1) / (5 - 1) => 1.6 = I - 1 => I = 2.6
Using the depression in freezing point formula (ΔTf = i·Kf·m), and equating the ΔTf for two different solutions:
ΔTf (K? [Fe (CN)? ]) = ΔTf (A)
i? ·Kf·m? = i? ·Kf·m?
(2.6) * [ (18.1 / M) / (100-18.1)/1000] = (1) * [ (w? /M? ) / (W? /1000)]
The problem simplifies to finding the molar mass M of solute A:
2.6 * [18.1 / (M * 81.9)] * 1000 = [w? /M? ] * [1000/W? ]
Assuming the secon
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers