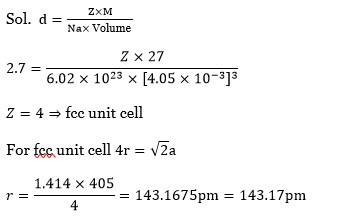Solid State
Get insights from 127 questions on Solid State, answered by students, alumni, and experts. You may also ask and answer any question you like about Solid State
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
ρ = M/V
∴ 6.17 = (2 x MX? ) / (N? x (300 x 10? ¹? )³)
Solving MX? = 50 g
∴ No. of molecules in 200 g
= (200/50) x N? = 4 N?
New answer posted
5 months agoContributor-Level 10
In the ccp lattice of oxide ions effective number of O? ² ions = 8 *? + 6 * ½ = 4
In the ccp lattice,
No. of octahedral voids = 4
No. of tetrahedral voids = 8
Given M? atoms occupies 50% of octahedral voids and M? atoms occupies 12.5 of tetrahedral voids
No. of M? metal atoms = 4 *? /? = 2
No. of M? metal atoms = 8 * ¹²·? /? = 1
∴ Formula of the compound = (M? )? (M? )O?
∴ Oxidation states of metals M? & M? respectively are +2 and +4 .
New answer posted
5 months agoContributor-Level 9
For AB? compound possible geometry are
S. No. | Bond pair | Lone pair | Total | Hybridisation | Geometry | Polarity |
1 | 4 | 0 | 4 |
| Tetrahedral | non polar |
2 | 4 | 1 | 5 |
| Sea-saw | Polar |
3 | 4 | 2 | 6 |
| Square Planar | non polar |
Square pyramidal can be polar due to lone pair moment as the bond pair moments will get cancelled out.
New answer posted
5 months agoContributor-Level 10
In FCC octahedral voids are present at the edge centers and body center
Consider a diagonal projected form edge centre passing through the body centre
Distance between octahedral voids = √2a/2 = a/√2
New answer posted
5 months agoContributor-Level 10
In Monoclinic crystal system, a ≠ b ≠ c and α = 90°, γ = 90° and β=120°.
New answer posted
6 months agoContributor-Level 10
Effective no. of octahedral voids in a lattice = n
Effective no. of lattice point in a lattice = n
Ratio = n/n = 1
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers