Solutions
Get insights from 202 questions on Solutions, answered by students, alumni, and experts. You may also ask and answer any question you like about Solutions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
let the molar masses of AB2 and AB4 be x and y respectively.
Molar mass of benzene (C6H6) = 12 * 6 + 1 * 6 = 78 g/mol
Moles of benzene = mass/molar mass = 20/78
n = 0.256mol
⇒ ΔTf = 2.3 K
Kf = 5.1K kg mol-1
For AB2
Applying the formula: ΔTf = Kf * M
Where
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ 2.3 = 5.1 * M1
⇒ M1 = 0.451mol/kg
For AB4
Applying the formula: ΔTf = Kf * M
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ 1.3 = 5.1 * M2
⇒ M2 = 0.255mol/kg
M1 = moles of solute/mass of solvent (in kg)
M1 = 1/x / 0.02 = 1 / 0.02x = 0.451
⇒ X = 110.86g
M2
New answer posted
9 months agoContributor-Level 10
5% solution means 5g of cane sugar is present in 100g of solution
Freezing point of solution = 271k
Freezing point of pure water = 273.15k
Molar mass of cane sugar (C12H22O11) = 12 * 12 + 1 * 22 + 16 * 11 = 342g
Moles of cane sugar = mass/molar mass = 5/342
⇒ n = 0.0146mol
Molality of solution = moles of solute/mass of solvent (in kg)
⇒ M = 0.0146/0.095
⇒ Molality = 0.154M
Depression in freezing point = ΔTf = 273.15-271 = 2.15k
Applying the formula: ΔTf = Kf * M
Where
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ Kf = 2.15/0.154
⇒ Kf = 13.96k kg mol-1
Second condition: mass of glucose = 5g
Mola
New answer posted
9 months agoContributor-Level 10
Given: mass of solute = 30g
Let the molar mass of solute be x g and vapour pressure of pure water at 298k be P1 ?
Mass of water(solvent) = 90g
Molar mass of water = H2O = 1 * 2 + 16 = 18g
Moles of water = mass of water/molar mass
⇒ n = 90/18 moles
⇒ n = 5moles
Molar fraction of solute,
x2 = moles of solute / moles of solute + moles of octane
x2 = (30/x) / (30/x) + 5
x2 = 30 / 30+5x
Vapour pressure of solution (p1) = 2.8kpa
Applying the formula:

According to second condition when we add 18g of water to solution vapour pressure becomes 2.9kpa
Moles of water = mass/molar mass
⇒ n = 90 + 18/18
⇒ n = 6moles
Molar fraction of solute,
x2 = mo
New question posted
9 months agoNew question posted
9 months agoNew question posted
9 months agoNew answer posted
9 months agoContributor-Level 10
Given:
Molar mass of non-volatile solute = 40g
Let no. of moles of solute be n.
Mass of octane = 114g
Molar mass of octane (C8H18) = 12 * 8 + 1 * 18 = 114g/mol
Moles of octane = given mass/molar mass
⇒ n = 114/114 moles
⇒ n = 1 mole
Molar fraction of solute,
x2 = moles of solute / moles of solute + moles of octane
⇒ x2 = n/n + 1
Let the vapour pressure of original solvent (without solute) be p1?
Accordingly after addition of solute vapour pressure of solution reduces to 80% i.e.
0.8 p1? = p1
Applying the formula:

⇒ n/n + 1 = 0.2
⇒ 0.2n + 0.2 = n
⇒ n = 0.25 moles
Hence, mass of solute is:
moles = given mass/molar mass
⇒ 0.25moles = ma
New answer posted
9 months agoContributor-Level 10
Given: 1 molal solution means 1 mole of solute present in 1000g of water solvent)
Molecular weight of water = H2O = 1 * 2 + 16 = 18g/mol
No. of moles of water, n = given mass /molecular weight
⇒ n = 1000/18 = 55.56 gmol-1
Mole fraction of solute in solution, x2 = moles of solute/ (moles of solute + moles of water)
⇒ x2 = 1/ (1 + 55.56)
⇒ x2 = 0.0177
Given vapour pressure of pure water at 300k is 12.3 kpa
Apply the formula:
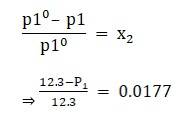
⇒ P1 = 12.0823kpa
which is the vapour pressure of the solution.
New answer posted
9 months agoContributor-Level 10
Given: Temperature = 373k
Vapour pressure of pure heptane (p10 ) = 105.2 kpa and that of octane (p20 ) = 46.8 kpa
Mass of heptane = 26 g
Mass of octane = 35 g
Molecular weight of heptane = C7H16 = 12 * 7 + 1 * 16 = 100 gmol-1
Molecular weight of octane = C8H18 = 114 gmol-1
Moles of heptane, n1 = given mass /molecular weight = 26/100
⇒ n1 = 0.26mol
Moles of octane, n2 = given mass /molecular weight = 35/114
⇒ n2 = 0.307mol
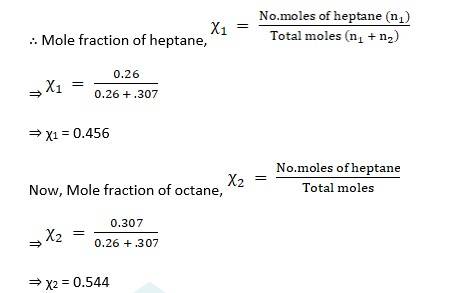
∴ Partial pressure of heptane, p1 = χ1 * p10
⇒ p1 = 0.456 * 105.2 = 47.97kpa
∴ Partial pressure of octane, p1 = χ2 * p20
⇒ p2 = 0.544 * 46.8 = 25.46 kpa
∴ Total pressure exerted by solution = p1 + p2
= 47.97 + 25.46
=
New answer posted
9 months agoContributor-Level 10
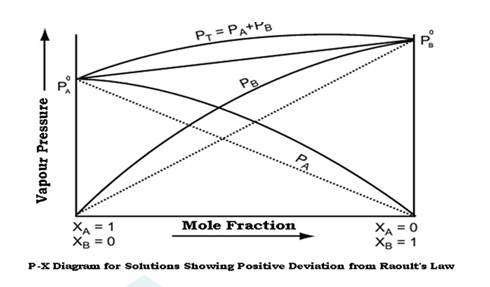
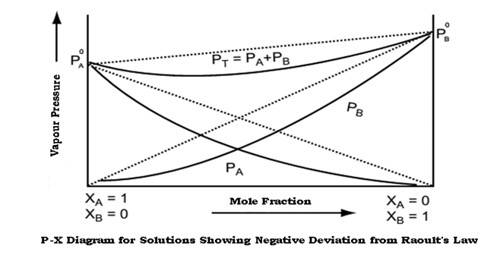
Raoult's law states that at a given temperature, the vapour pressure of a solution containing non volatile solute is directly proportional the mole fraction of the solvent.
Non ideal solutions show positive and negative deviations from ideal behaviour.
Non ideal solutions showing positive deviations from Raoult's law-
A solution is said to show positive deviation from Raoult's Law when at any composition, its vapour pressure is higher than that given by Raoult's Law.
The positive deviation is shown by those liquid pairs in which the A-B molecular interaction forces are weaker than the corresponding A-A or B-B molecular interaction forces.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers