Solutions
Get insights from 202 questions on Solutions, answered by students, alumni, and experts. You may also ask and answer any question you like about Solutions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
Given:
Mass of ethane in solution A = 6.56 * 10–3 g
Partial pressure of solution A = 1 bar
Mass of ethane in solution B = 5.00 * 10–2 g
To find: Partial Pressure of gas
Formula:
By Henry's law:
Mass of dissolved gas M = k * P Where
k = proportionality constant
P = Partial Pressure
Solution:
⇒ M1 = k * P1. [1]
⇒ M2 = k * P2. [2]
Dividing the [2] by [1], we get
M2/M1 = P2/P1
P2 = M2 X P1 / M1
P2 = 5 X 10–2 X 1 / 6.56 X 10–3
P2 = 7.62 bar
Therefore the partial pressure of the gas is 7.62 bar.
New answer posted
9 months agoContributor-Level 10
The solubility of a gas in water depends on following three parameters:
- Nature of gas
- Temperature
- Pressure
The solubility decreases with increase in temperature. Temperature and pressure follow inverse proportionality. So solubility increases with increase with pressure. A quantitative relation between pressure and solubility of a gas in a solvent was given by W. Henry [1803]. This relationship is known as Henry's law.
Statement:
Henry's law can be expressed as follows.
At constant temperature, the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
Mathematically,
Solubility? Pressure of the gas
Some of the imp
New answer posted
9 months agoContributor-Level 10
Whenever a gas is dissolved in a liquid, a small amount heat is liberated in the process. So dissolving a gas in liquid is overall an exothermic process.
So according to the LeChatelier principle, whenever the temperature is increased for a reaction which is exothermic in nature, the equilibrium shifts backwards and the reaction proceeds in backward direction that means the solution gets dissociated and will give off gas and hence solubility of gas decreases.
So with the increase in temperature, the solubility of the gases in liquids decreases.
New answer posted
9 months agoContributor-Level 10
The lower members of alcohols are completely miscible [highly soluble] with water but the solubility decreases with increase in the molecular weight. The lower members of the alcohol group have the capability to form intermolecular hydrogen bonding with water molecules as alcohols are polar molecules in nature.
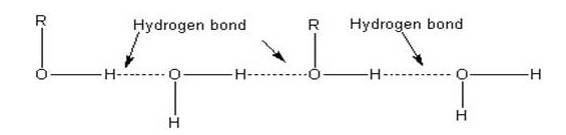
Alkyl groups are hydrophobic [prevents formation of hydrogen bonds with water] in nature. In lower alcohols, the alkyl group is small and the –OH group of alcohol is effective in making hydrogen bonds with water.
But with the increase in the size of alkyl group, the hydrophobic [water hating] nature of alkyl group dominates over
New answer posted
9 months agoContributor-Level 10
Given:
Level of contamination = 15 ppm [by mass]
To find: Mass Percentage and Molality
Formula:
Molality = Number of moles of solute / Mass of solvent in kg
Mass Percentage of Solute = Mass of solute / Mass of solution X 100
Solution:
Calculation of Mass Percentage:
15 ppm means 15 parts of Chloroform in 106 parts of drinking water
⇒ Mass Percentage = Mass of choloroform / Total mass X 100
= 15 / 106 X 100
= 1.5 * 10-3
Calculation of Molality:
⇒ Molecular Mass of Chloroform, CHCl3 = [12] + [1] + [35.5 * 3]
= 119.5 g
⇒ Number of Moles of Chloroform = [15 / 119.5]
= 0.1255 moles
Molality = Number of moles of solute / Mass of solvent in
New answer posted
9 months agoContributor-Level 10
Given:
Mass of ethylene glycol (C2H6O2) = 222.6 g
Mass of water = 200 g
Density, d = 1.072 g/ml
To find: Molality and Molarity of solution
Formula:
Molality = Number of moles of solute/ Mass of solvent in kg
Molarity, Mo = number of moles of solute/ volume of solution in litres
Density, d = Mass (M) / volume (V)
Calculation of Molality:
⇒ Molecular Mass of ethylene glycol (C2H6O2) = [12 * 2] + [6 * 1] + [16 * 2]
= 24 + 6 + 32
= 62 g
⇒ Number of moles of ethylene glycol (C2H6O2) = [222.6/62]
= 3.59 moles
⇒ Mass of water = 200 g
⇒ Molality = numebr of moles of solute/ Mass of solvent in kg
= 3.59 / 200 X 1000
= 17.95 m
Calculation of Molarity:
⇒ T
New answer posted
9 months agoContributor-Level 10
Given:
Molarity of HCl, = 0.1 M
Mass of Mixture = 1g
To find: Volume of HCl to react completely with mixture
Formula:
Molarity, Mo = number of moles of solute/ Volume of solution in litres
Solution:
Calculation of Amount of each component in mixture:
⇒ Let the amount of Na2CO3 be X g
⇒ And Let amount of NaHCO3 be [1-X] g
⇒ Molecular Weight of Na2CO3 = [23 * 2] + [12] + [3 * 16]
= 106 g
⇒ Molecular Weight of NaHCO3 = [23] + [1] + [12] + [3 * 16]
= 84 g
⇒ Number of moles of NaHCO3 = 1-x / 84
⇒ Number of moles of Na2CO3 = x / 106
Now it is given in the question that the mixture is equimolar, so
⇒ Number moles of Na2CO3 = Number of moles
New answer posted
9 months agoContributor-Level 10
Given:
Concentration of Nitric Acid, HNO3 = 68%
Density of solution, d = 1.504 g/ml
To find: Molarity, Mo
Formula:
Density, d = Mass (M) / volume (V)
Molarity, Mo = Number of moles of solute/ Volume of solution in litres
Solution:
68% of Nitric acid by mass in aqueous solution means that 68g [68 * 100]/100] of Nitric acid present in 100g of solution.
⇒ Molecular mass of Nitric Acid, HNO3 = [1 * 1] + [1 * 14] + [16 * 3]
= 63g
⇒ Number of moles of Nitric Acid = [68/63]
= 1.079 moles
⇒ Given Density, d = 1.504 g/ml
⇒ Volume, v = [100/1.504]
= 66.489 ml
⇒ Molarity, Mo = [1.079/66.489] * 1000
= 16.23 M
Therefore the molarity of the sample is
New answer posted
9 months agoContributor-Level 10
As the name signifies, a solid solution is one in which solvent is solid.So considering this aspect absorption of hydrogen over platinum or palladium is an example of such solution. Platinum or palladium is used as a catalyst in hydrogenation processes.
New question posted
9 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
