- Solid States Questions and Answers
- JEE Mains 2022
Solid States Questions and Answers
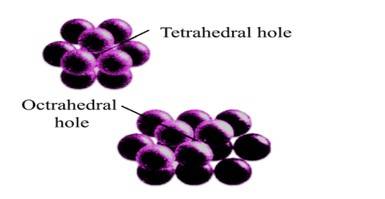
| 1. With the help of a labelled diagram show that there are four octahedral voids per unit cell in a cubic close packed structure. |
| Ans: When any atom is surrounded by six atoms it creates an octahedral void. In fcc, body centre is surrounded by six atoms present at the face centre. And hence one octahedral void is present at the body centre of each unit cell. No. of octahedral void at the centre of 12 edge = 12 × = 3 No. of octahedral void at body centre = 1 Total no. of octahedral void in ccp = 3+1+4 Location of octahedral voids per unit cell of ccp or fcc lattice (a) at body centre of the cube and (b) at the centre of each edge (only one such void is shown) |
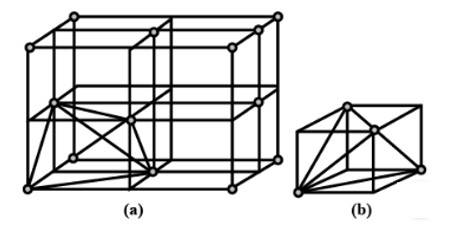
| 2. Show that in a cubic close packed structure, eight tetrahedral voids are present per unit cell. |
| Ans: A ccp structure unit cell is divided into 8 small cubes. Each small cube has atoms at alternate corners as shown in the figure. In all, each small cube has 4 atoms. When these are joined to one another, they make a regular tetrahedron. Thus, there is one tetrahedral void in each small cube and 8 tetrahedral voids in total. Each of the eight small cubes has one void in one unit cell of ccp structure. We know that ccp structure has four atoms per unit cell. Thus, the number of tetrahedral voids is twice the number of atoms. |
| 3. How does doping increase the conductivity of semiconductors? |
| Ans: The conductivity of a semiconductor is very low and it can be increased by adding small impurities and this process is known as doping.
Doping can be done with an impurity which is electron rich or electron deficient- n-type semiconductors- Si or Ge (group-14 elements) are doped with electron rich impurity (group-15 elements like P or As) and are known as n-type semiconductors. In them the conductivity is due to the extra electron or delocalized electron.
When intrinsic semiconductors like Si or Ge are doped with pentavalent elements like P or As , they occupy some of the lattice sites in silicon or germanium crystal . Four out of five electrons are used in formation of four covalent bonds with four neighbouring silicon atoms. The fifth electron is extra and gets delocalised. These delocalised electrons increase the conductivity of doped silicon ( or germanium).
p-type semiconductors- Si or Ge (group-14 elements) doped with electron deficient impurity (group 13 elements like B or Al or Ga) and are termed as p-type semiconductors. Here the conductivity is due to the positively charged electron holes.
In this case the doping of intrinsic semiconductors like silicon or germanium with trivalent elements like B / In / Ga , three out of four electrons in silicon or germanium form bonds with doping impurity ( i.e.. B/ In / Ga ). The fourth electron remains unbonded . The place where the fourth valence electron is missing is known as electron hole or electron vacancy. An electron from a neighbouring atom can come and fill up the electron hole, but in doing so it would leave an electron hole at its original position. If this happens it would appear that the electron hole has moved in a direction opposite to that of the electron that filled it. Under the influence of electric field electrons would move towards the positively charged plate through electron holes, but it would appear as if electrons were positively charged and are moving towards the negatively charged plate.
|
| 4. A sample of ferrous oxide has the actual formula Fe0.93 O1.00. In this sample, what fraction of metal ions are Fe2+ ions? What type of non stoichiometric defect is present in this sample? |
| Ans: Let the formula of sample be (Fe2+)x(Fe3+) yO From the formula of the compound the equation can be formed as- x+ y= 0.93 ... (i) Total positive charge on ferrous and ferric ions should balance the two units of negative charge on oxygen. Hence, 2x+ 3y= 2 ... (ii) ⇒ x+ y=1....(iii) On subtracting equation (i) from equation (iii) we have y-y=1 - 0.93 12y=0.07 y= 0.14 On putting the value of y in equation (i) we get, x+ 0.14 = 0.93 ⇒x = 0.93 – 0.14 ⇒x = 0.79 Fraction of Fe2+ ions present in the sample is = 0.81 Metal deficiency defect is found to be present in the sample as iron is less in amount than that required for stoichiometric composition. |
Commonly asked questions
19. Which of the following is true about the value of refractive index of quartz glass?
(i) Same in all directions
(ii) Different in different directions
(iii) Cannot be measured
(iv) Always zero
19. Option (i) Same in all directions is correct since quartz glass is an amorphous solid showing isotropic properties and hence exhibits the same values of refractive index when measured along different directions.
18. Which of the following arrangements shows schematic alignment of magnetic moments of antiferromagnetic substances?
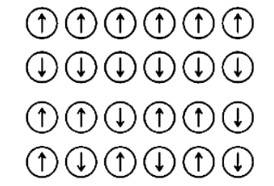
18. Option (iv) is correct since in antiferromagnetic substances the domains are oppositely oriented and hence they cancel out each other's magnetic moments.
21. The sharp melting point of crystalline solids is due to ___________.
(i) a regular arrangement of constituent particles observed over a short distance in the crystal lattice.
(ii) a regular arrangement of constituent particles observed over a long distance in the crystal lattice.
(iii) same arrangement of constituent particles in different directions.
(iv) different arrangement of constituent particles in different directions.
21. Option (ii) a regular arrangement of constituent particles observed over a long distance in the crystal lattice is correct since the regularity of the crystalline lattice creates local environments that are the same and hence crystals exhibit sharp melting point.
23. Which of the following is a network solid?
(i) SO2 (Solid)
(ii) I2
(iii) Diamond
(iv) H2O (Ice)
23. Option (iii) Diamond is correct since in diamond, the carbon atoms are held together by strong covalent bonds. It is a giant molecule. Thus, it is a solid network.
29. The lattice site in a pure crystal cannot be occupied by _________.
(i) Molecule
(ii) Ion
(iii) Electron
(iv) Atom
29. Option (iii) electron is correct since lattice sites can only be occupied by electrons when there is imperfection in a solid and do not occur in pure crystals. Hence, the lattice site in a pure crystal cannot be occupied by electrons.
49. In the cubic close packing, the unit cell has ________.
(i) 4 tetrahedral voids each of which is shared by four adjacent unit cells.
(ii) 4 tetrahedral voids within the unit cell.
(iii) 8 tetrahedral voids each of the which is shared by four adjacent unit cells.
(iv) 8 tetrahedral voids within the unit cells.
49. Option (iv) 8 tetrahedral voids within the unit cells is correct since in ccp structure there are 4 atoms per unit cell and the number of tetrahedral voids is twice the number of atoms (i.e. eight tetrahedral voids per unit cell of cubic close packing.
50. The edge lengths of the unit cells in terms of the radius of spheres constituting fcc, bcc and simple cubic unit cell are respectively________.
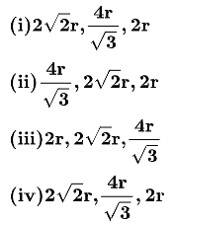
50. Option (i) , ,2r
In FCC crystal - the atoms touch each other at the face diagonals. If a is the edge length of the cube and r is the radius of the atom
Therefore,
a =
In a simple cubic crystal - The atoms at the corners touch each other.
Therefore, a = 2r
66. Frenkel defect is also known as ________.
(i) Stoichiometric defect
(ii) Dislocation defect
(iii) Impurity defect
(iv) Non-stoichiometric defect
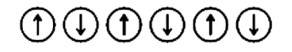
66. Options (i) and (ii) are correct since in this defect an atom is displaced from its lattice point to an interstitial site and thus creating a vacancy at the lattice point. This usually occurs when the size of anion is quite large as compared to that of the cation due to which it leads to the dislocation of the cation to the interstitial site and hence it is known as dislocation defect and during this defect there is no change in the stoichiometry of the crystal so, it is also known as stoichiometric defect.
67. Which of the following defects decrease the density?
(i) Interstitial defect
(ii) Vacancy defect
(iii) Frankel defect
(iv) Schottky defect
67. Options (ii) and (iv) are correct since Vacancy and Schottky, both the defects lead to the decrease in the density while in the Frenkel and Interstitial defect there is no change in the density of the substance.
76. Assertion: The packing efficiency is maximum for the fcc structure.
Reason: The coordination number is 12 in fcc structures.
76. Option (ii) Assertion and reason both are correct statements, but reason is not correct explanation for assertion is the answer since in fcc structure the number of atoms present = 4 per unit cell which provides a maximum packing efficiency of 74%.
77. Assertion: Semiconductors are solids with conductivities in the intermediate range from 10−6 104 ohm−1 m1.
Reason: Intermediate conductivity in semiconductor is due to partially filled valence band.
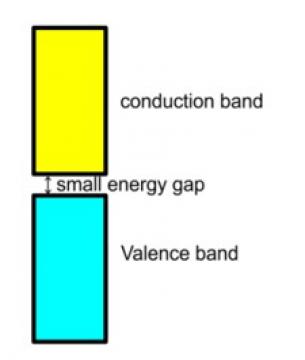
77. Option (iii) Assertion is correct statement, but reason is wrong statement is the answer since intermediate conductivity in semiconductor is due to the small energy gap between valence band and conduction band and hence the assertion is correct, but the reason is wrong.
1. With the help of a labelled diagram show that there are four octahedral voids per unit cell in a cubic close packed structure.
1. When any atom is surrounded by six atoms it creates an octahedral void. In fcc, body centre is surrounded by six atoms present at the face centre. And hence one octahedral void is present at the body centre of each unit cell.
No. of octahedral void at the centre of 12 edge = 12 * = 3
No. of octahedral void at body centre = 1
Total no. of octahedral void in ccp = 3+1+4
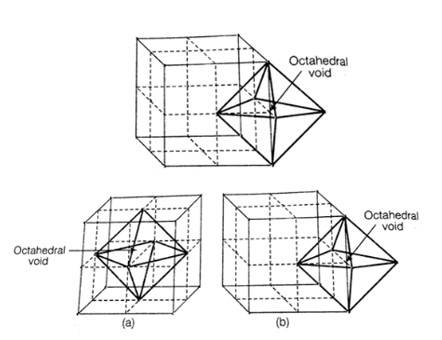
Location of octahedral voids per unit cell of ccp or fcc lattice (a) at body centre of the cube and (b) at the centre of each edge (only one such void is shown)
2. Show that in a cubic close packed structure, eight tetrahedral voids are present per unit cell.
2. A ccp structure unit cell is divided into 8 small cubes. Each small cube has atoms at alternate corners as shown in the figure. In all, each small cube has 4 atoms. When these are joined to one another, they make a regular tetrahedron. Thus, there is one tetrahedral void in each small cube and 8 tetrahedral voids in total. Each of the eight small cubes has one void in one unit cell of ccp structure. We know that ccp structure has four atoms per unit cell. Thus, the number of tetrahedral voids is twice the number of atoms.
3. How does doping increase the conductivity of semiconductors?
3. The conductivity of a semiconductor is very low and it can be increased by adding small impurities and this process is known as doping.
Doping can be done with an impurity which is electron rich or electron deficient-
n-type semiconductors- Si or Ge (group-14 elements) are doped with electron rich impurity (group-15 elements like P or As) and are known as n-type semiconductors. In them the conductivity is due to the extra electron or delocalized electron.
When intrinsic semiconductors like Si or Ge are doped with pentavalent elements like P or As, they occupy some of the lattice sites in silicon or germanium crystal . Four out of five electrons are used in formation of four covalent bonds with four neighbouring silicon atoms. The fifth electron is extra and gets delocalised. These delocalised electrons increase the conductivity of doped silicon ( or germanium).
p-type semiconductors- Si or Ge (group-14 elements) doped with electron deficient impurity (group 13 elements like B or Al or Ga) and are termed as p-type semiconductors. Here the conductivity is due to the positively charged electron holes.
In this case the doping of intrinsic semiconductors like silicon or germanium with trivalent elements like B / In / Ga, three out of four electrons in silicon or germanium form bonds with doping impurity ( i.e. B/ In / Ga ). The fourth electron remains unbonded. The place where the fourth valence electron is missing is known as electron hole or electron vacancy.
An electron from a neighbouring atom can come and fill up the electron hole, but in doing so it would leave an electron hole at its original position. If this happens it would appear that the electron hole has moved in a direction opposite to that of the electron that filled it. Under the influence of electric field electrons would move towards the positively charged plate through electron holes, but it would appear as if electrons were positively charged and are moving towards the negatively charged plate.
4. A sample of ferrous oxide has the actual formula Fe0.93 O1.00. In this sample, what fraction of metal ions are Fe2+ ions? What type of non stoichiometric defect is present in this sample?
4. Let the formula of sample be (Fe2+)x (Fe3+) yO
From the formula of the compound the equation can be formed as-
x+ y= 0.93 . (i)
Total positive charge on ferrous and ferric ions should balance the two units of negative charge on oxygen. Hence,
2x+ 3y= 2 . (ii)
⇒ x+ y=1. (iii)
On subtracting equation (i) from equation (iii) we have
y-y=1 - 0.93
12y=0.07
y= 0.14
On putting the value of y in equation (i)
we get, x+ 0.14 = 0.93
⇒x = 0.93 – 0.14
⇒x = 0.79
Fraction of Fe2+ ions present in the sample is = 0.81
Metal deficiency defect is found to be present in the sample as iron is less in amount than that required for stoichiometric composition.
5. Why are liquids and gases categorised as fluids?
5. The liquids and gases both are categorised as fluids as they show the ability to flow and also their molecules can move past one another freely.
6. Why are solids incompressible?
6. In solids the constituent particles are found to possess fixed positions and can only oscillate about their mean positions and hence they are said to be incompressible and rigid.
7. Inspite of long-range order in the arrangement of particles why are the crystals usually not perfect?
7. Crystals have long range ordered arrangement of their constituent particles but usually these crystals are not perfect as during the process of crystallisation some deviations as compared to such ideal arrangement set in depending upon the rate of cooling or presence of impurities in solution also this process occurs at such a rate that the constituent particles may not get the sufficient time to arrange themselves in a perfect order and hence these deviations or irregularities in arrangement is being termed as defects or imperfections. Therefore, crystals are usually not perfect.
8. Why does table salt, NaCl, sometimes appear yellow in colour?
8. Yellow colour in NaCl is due to the defect called as metal excess defect. In this defect anionic vacancies get created due to the diffusion of Cl-ions to the surface of the crystal and there after unpaired electrons occupy anionic sites. These sites are known as F-centres. The electrons at F-centres then absorb energy from the visible region and undergo excitation which makes the crystal appear yellow.
9. Why is FeO (s) not formed in stoichiometric composition?
9. In FeO crystal, some of the Fe2+ ions are replaced by Fe3+ ions i.e., 3Fe2+ ions are replaced by 2Fe3+ ions to make up for the loss of positive charge. As a result of which it leads to lesser amount of metal as compared to the stoichiometric proportion.
10. Why does white ZnO (s) becomes yellow upon heating?
10. The ZnO crystal becomes yellow oh heating because of the metal excess defect which is caused due to the presence of extra cations at the interstitial sites and on heating this white crystal it loses oxygen and turns yellow. The reaction involved is given as-
ZnO Zn2+ + O2 + 2e-
Here the excess of Zn2+ ions move to the interstitial sites and electrons to neighbouring interstitial sites.
11. Why does the electrical conductivity of semiconductors increase with rise in temperature?
11. In semiconductors the gap between conduction band and valence band is small and hence some of the electrons from the valence band can easily jump to the conduction band and shows some conductivity but with rise in the temperature more of the electrons gets jump to the conduction band and thus, their electrical conductivity increases with rise in the temperature.
12. Explain why conductivity of germanium crystals increases on doping with gallium.
12. When tetravalent germanium is doped with trivalent gallium then some of the positions of the lattice of germanium gets occupied by the gallium. Since the gallium atom has only three valence electrons, the fourth valency of the nearby germanium atom does not get satisfied and hence this place remains vacant. This place is deficient of electrons and is therefore called an electron hole or electron vacancy.
Now, the electron from the neighbouring atom comes and fills the gap and leads to the formation of a hole in its original position. Under the influence of electric fields, the electrons move towards the positively charged plates through these holes and lead to the conduction of electricity while the holes appear to move towards the negatively charged plates.
13. In a compound, nitrogen atoms (N) make cubic close packed lattice and metal atoms (M) occupy one-third of the tetrahedral voids present. Determine the formula of the compound formed by M and N?
13. In ccp lattice the number atoms per unit cell = 4
The number of tetrahedral voids is given as = 2 (n) = 2 x 4 = 8
Only one-third of tetrahedral voids are occupied by metal M so, the ratio of atoms of element M to that of element N = 1/3 (8) : (4) = 2 : 3
or M : N = 2 : 3
Hence, the formula of the compound is M2N3.
14. Under which situations can an amorphous substance change to crystalline form?
14. On heating the amorphous substance it gets changed to the crystalline form at some temperature. This is due to the process called crystallization. As on heating at some temperature it may become crystalline since slow heating and cooling over a longer period of time makes these changes.
15. Which of the following conditions favours the existence of a substance in the solid state?
(i) High temperature
(ii) Low temperature
(iii) High thermal energy
(iv) Weak cohesive forces
15. Option (ii) Low temperature is correct since at sufficiently low temperature, the thermal energy is low, so the intermolecular forces bring the molecules of a substance closer so that they cling to one another and occupy fixed positions. They keep on vibrating about their fixed positions. Such conditions favours the existence of the substance in solid state.
16. Which of the following is not a characteristic of a crystalline solid?
(i) Definite and characteristic heat of fusion.
(ii) Isotropic nature.
(iii) A regular periodically repeated pattern of arrangement of constituent particles in the entire crystal.
(iv) A true solid
16. Option (ii) Isotropic nature is correct since crystalline solids exhibit anisotropic properties like refractive index, electrical resistance etc. Since these are found to have different values when measured along different directions in the same crystal and hence they are not isotropic in nature.
17. Which of the following is an amorphous solid?
(i) Graphite (C)
(ii) Quartz glass (SiO2)
(iii) Chrome alum
(iv) Silicon carbide (SiC)
17. Option (ii) Quartz glass (SiO2) is correct since quartz glass (SiO2)is amorphous in nature as there is no long range ordered arrangement of the constituent particles being present in it and hence it is an amorphous solid.
20. Which of the following statement is not true about amorphous solids?
(i) On heating they may become crystalline at certain temperature.
(ii) They may become crystalline on keeping for long time.
(iii) Amorphous solids can be moulded by heating.
(iv) They are anisotropic in nature.
20. (iv) They are anisotropic in nature since amorphous solid shows isotropic properties as they exhibit same values of the properties like refractive index, electrical resistance when measured along different directions.
22. Iodine molecules are held in the crystals lattice by ____________.
(i) London forces
(ii) dipole-dipole interactions
(iii) covalent bonds
(iv) coulombic forces
22. Option (i) London forces is correct since iodine molecules are nonpolar and covalent in nature. These molecules are found to be electrically symmetrical and have no dipole moment. The molecules in a crystal lattice of iodine are thus attracted together by weak London forces.
24. Which of the following solids is not an electrical conductor?
(A) Mg (s)
(B) TiO (s)
(C) I2(s)
(D) H2O (s)
(i) (A) only
(ii) (B) Only
(iii) (C) and (D)
(iv) (B), (C) and (D)
24. Option (iii) (C) and (D) is correct since Iodine is a non-polar molecular solid which acts as an insulator while H2O is a hydrogen bonded molecular solid which also acts as an insulator. Whereas Mg is a metal and acts as a conductor in solid and in its molten state and TiO is also an ionic solid which acts as an insulator in solid state but acts as a conductor in molten and in aqueous solutions.
25. Which of the following is not the characteristic of ionic solids?
(i) Very low value of electrical conductivity in the molten state.
(ii) Brittle nature.
(iii) Very strong forces of interactions.
(iv) Anisotropic nature.
25. Option (i) Very low value of electrical conductivity in the molten state is correct since in molten state, the ionic solids possess free ions which help in the conduction of electricity. Due to the availability of these free ions the electrical conductivity of ionic compounds in molten state is found to be very high.
12. Graphite is a good conductor of electricity due to the presence of __________.
(i) Lone pair of electrons
(ii) Free valence electrons
(iii) Cations
(iv) Anions
26. (ii) Free valence electrons
27. Which of the following oxides behaves as conductor or insulator depending upon temperature?
(i) TiO
(ii) SiO2
(iii) TiO3
(iv) MgO
27. Option (iii) TiO3 is correct since certain metal oxides such as VO, VO2, VO3and TiO3 show either metallic or insulating properties depending upon the temperature.
28 Which of the following oxides shows electrical properties like metals ?
(i) SiO2
(ii) MgO
(iii) SO2
(iv) CrO2
28. Option (iv) CrO2 is correct since it shows electrical conductivity similar to the metals.
30. Graphite cannot be classified as __________.
(i) Conducting solid
(ii) Network solid
(iii) Covalent solid
(iv) Ionic solid
30. Option (iv) ionic solid is correct since in graphite each atom is linked to the other through a covalent bond and hence it is a covalent solid and cannot fall under the category of ionic solid.
31. Cations are present in the interstitial sites in __________.
(i) Frenkel defect
(ii) Schottky defect
(iii) Vacancy defect
(iv) Metal deficiency defect
31. Option (i) Frenkel defect is correct since it is a type of defect in which an atom is dislocated from its lattice position to an interstitial site.
32. Schottky defect is observed in crystals when __________.
(i) Some cations move from their lattice site to interstitial sites.
(ii) Equal number of cations and anions are missing from the lattice.
(iii) Some lattice sites are occupied by electrons.
(iv) Some impurity is present in the lattice.
32. Option (ii) equal number of cations and anions are missing from the lattice is correct since Schottky defect in crystals occurs when equal numbers of cations and anions are missing from the lattice.
33. Which of the following is true about the charge acquired by p-type semiconductors?
(i) Positive
(ii) Neutral
(iii) Negative
(iv) Depends on concentration of impurity
33. Option (ii) neutral is correct since p-type semiconductor mainly contains positively charged carriers ( holes ) which are able to move freely. It is still considered to be neutral as the fixed acceptor atoms having accepted electrons are negative.
34. To get a n-type semiconductor from silicon, it should be doped with a substance with valence__________.
(i) 2
(ii) 1
(iii) 3
(iv) 5
34. Option (iv) 5 is correct since silicon is doped with a pentavalent substance like Phosphorus or Arsenic so, four out of five electrons are used in the formation of four covalent bonds with four of its neighbouring silicon atoms. The fifth electron is extra and becomes delocalised. These delocalised electrons increase the conductivity of doped silicon.
21. The total number of tetrahedral voids in the face centred unit cell is __________.
(i) 6
(ii) 8
(iii) 10
(iv) 12
35. Option (ii) 8 is correct since in face centred cubic unit cell the number of atoms per unit cell is 4 atoms and hence the number of tetrahedral voids in fcc unit cell is found to be 8
22. Which of the following point defects are shown by AgBr(s) crystals?
(A) Schottky defect
(B) Frenkel defect
(C) Metal excess defect
(D) Metal deficiency defect
(i) (A) and (B)
(ii) (C) and (D)
(iii) (A) and (C)
(iv) (B) and (D)
36. Option (i) (A) and (B) is correct since AgBr shows both Frenkel as well as Schottky defects.
37. In which pair most efficient packing is present?
(i) Hcp and bcc
(ii) Hcp and ccp
(iii) Bcc and ccp
(iv) Bcc and simple cubic cell
37. Option (ii) hcp and ccp is correct since in hcp and ccp the packing efficiency is 74% ( maximum ), hence this pair of packing is considered as the most efficient packing.
38. The percentage of empty space in a body centred cubic arrangement is ________.
(i) 74
(ii) 68
(iii) 32
(iv) 26
38. Option (iii) 32 is correct since the packing efficiency in bcc arrangement is 68%, therefore the percentage empty space can be calculated as – 100 - 68 = 32
39. Which of the following statement is not true about the hexagonal close packing?
(i) The coordination number is 12.
(ii) It has 74% packing efficiency.
(iii) Tetrahedral voids of the second layer are covered by the spheres of the third layer.
(iv) In this arrangement spheres of the fourth layer are exactly aligned with those of the first layer.
39. Option (iv) since in this arrangement the spheres of fourth layer are exactly aligned with those of the first layer is correct since in hexagonal close packed structure there is ABAB.type arrangement which means that in this case spheres of third layer are exactly aligned with that of the first layer. Hence, the pattern of spheres is repeated in alternate layers. Thus, the statement at (iv) is not true about hexagonal close packing.
40. In which of the following structures coordination number for cations and anions in the packed structure will be same?
(i) Cl ion form fcc lattice and Na+ ions occupy all the octahedral voids of the unit cell.
(ii) Ca2+ions form fcc lattice and F ions occupy all the eight tetrahedral voids of the unit cell.
(iii) O2 ions form fcc lattice and Na+ions occupy all the eight tetrahedral voids of the unit cell.
(iv) S2ions form fcc lattice and Zn2− ions go into the alternate tetrahedral voids of the unit cell.
40. Option (i) Cl-ion form fcc lattice and Na+ ions occupy all octahedral voids of the unit cell is correct since In NaCl, chloride ion form fcc lattice and sodium ions occupy all the octahedral voids of the unit cell. The coordination number of both the cation and anion is found to be 6.
41. What is the coordination number in a square close packed structure in two dimensions?
(i) 2
(ii) 3
(iii) 4
(iv) 6
41. Option (iii) 4 is correct since in 2-D scope structure each sphere is in contact with four of its neighbours. Thus, its coordination number is found to be 4.
42. Which kind of defects are introduced by doping?
(i) Dislocation defect
(ii) Schottky defect
(iii) Frenkel defects
(iv) Electronic defects
42. Option (iv) Electronic defects is correct since doping can be done with an impurity which is electron rich or electron deficient as compared to the intrinsic semiconductor like Si or Ge . Such impurities lead to the electronic defects in them.
43. Silicon doped with electron-rich impurity forms ________.
(i) P-type semiconductor
(ii) N-type semiconductor
(iii) Intrinsic semiconductor
(iv) Insulator
43. Option (ii) n-type semiconductor is correct since silicon is doped with electron rich impurities like Phosphorus or Arsenic and this leads to the increase in the conductivity due to the negatively charged electron . Hence, silicon doped with electron rich impurity is termed as n -type semiconductor.
44. Which of the following statements is not true?
(i) Paramagnetic substances are weakly attracted by magnetic field.
(ii) Ferromagnetic substances cannot be magnetised permanently.
(iii) The domains in antiferromagnetic substances are oppositely oriented with respect to each other.
(iv) Pairing of electrons cancels their magnetic moment in the diamagnetic substances.
44. Option (ii) Ferromagnetic substances cannot be magnetised permanently is correct since ferromagnetic substances are those which are very strongly attracted by the magnetic field and can be magnetized permanently.
45. Which of the following is not true about the ionic solids?
(i) Bigger ions form the close packed structure.
(ii) Smaller ions occupy either the tetrahedral or the octahedral voids depending upon their size.
(iii) Occupation of all the voids is not necessary.
(iv) The fraction of octahedral or tetrahedral voids occupied depends upon the radii of the ions occupying the voids.
45. Option (iv) The fraction of octahedral or tetrahedral voids occupied depends upon the radii of the ions occupying the voids is not true since the occupation of the voids depends upon the stoichiometry of the compound instead of the radii of the ions.
46. A ferromagnetic substance becomes a permanent magnet when it is placed in a magnetic field because ________.
(i) All the domains get oriented in the direction of magnetic field.
(ii) All the domains get oriented in the direction opposite to the direction of magnetic field.
(iii) Domains get oriented randomly.
(iv) Domains are not affected by magnetic field.
46. Option (i) all the domains get oriented in the direction of magnetic field is correct since whenever a ferromagnetic substance is placed in a magnetic field it becomes a permanent magnet as all the domains get oriented in the directions of the magnetic field even after removal of the applied magnetic field.
47. The correct order of the packing efficiency in different types of unit cells is ________.
(i) fcc < bcc < simple cubic
(ii) fcc > bcc > simple cubic
(iii) fcc < bcc > simple cubic
(iv) bcc < simple > cubic
47. Option (ii) The correct order of the packing efficiency is fcc > bcc > simple cubic
Packing Efficiency (fcc) - 74%
Packing Efficiency (bcc) - 68%
Packing Efficiency (simple cubic) - 52%
48. Which of the following defects is also known as dislocation defect?
(i) Frenkel defect
(ii) Schottky defect
(iii) Non-stoichiometric defect
(iv) Simple interstitial defect
48. Option (i) Frenkel defect is correct since in this defect an atom is displaced from its lattice point to an interstitial site thus creating a vacancy at the lattice point. This usually occurs in those ionic crystals where the size of anion is quite large as compared to that of the cation. Here dislocation of atoms occurs and hence it is known as dislocation defect.
51. Which of the following represents correct order of conductivity in solids?
(i) κ metals >> κ insulators < κsemiconductors
(ii) κmetals << κinsulators < κsemiconductors
(iii) κmetals ? κsemiconductors > κinsulators = zero
(iv) κ metals < κ semiconductors > κinsulators ≠ zero
51. Option (i)? metals >>? insulators < ? semiconductors is correct since metals are the ones which shows the highest conductivity due to the presence of free electrons in them which helps in the conduction of electricity while semiconductors are the ones whose conductivity depends upon the temperature.
Since at normal temperatures, they act as insulators whereas if the temperature rises, the conductivity of the semiconductor also increases. Insulators are the ones which do not have free electrons in them. Due to which they act as poor conductors and hence their conductivity is the lowest.
52. Which of the following is not true about the voids formed in 3-dimensional hexagonal close packed structure?
(i) A tetrahedral void is formed when a sphere of the second layer is present above triangular void in the first layer.
(ii) All the triangular voids are not covered by the spheres of the second layer.
(iii) Tetrahedral voids are formed when the triangular voids in the second layer lie above the triangular voids in the first layer and the triangular shapes of these voids do not overlap.
(iv) Octahedral voids are formed when the triangular voids in the second layer exactly overlap with similar voids in the first layer.
52. Option (iii) and (iv) are correct since tetrahedral voids are formed when the triangular void in the second layer lie exactly above the triangular voids in the first layer and the triangular shape of these voids oppositely overlap while the octahedral voids are formed when triangular void of second layer is not exactly overlap with similar void in first layer. This can be shown as-
53. The value of magnetic moment is zero in the case of antiferromagnetic substances because the domains ________.
(i) Get oriented in the direction of the applied magnetic field.
(ii) Get oriented opposite to the direction of the applied magnetic field.
(iii) Are oppositely oriented with respect to each other without the application of magnetic field.
(iv) Cancel out each other’s magnetic moment.
53. Options (iii) and (iv) are correct since in anti-ferromagnetic substances the magnetic moments and domains are oppositely oriented, and they cancel out each other's magnetic moment. For example, the anti-ferromagnetic substance-MnO.
54. Which of the following statements are not true?
(i) Vacancy defect results in a decrease in the density of the substance.
(ii) Interstitial defects results in an increase in the density of the substance.
(iii) Impurity defect has no effect on the density of the substance.
(iv) Frankel defect results in an increase in the density of the substance.
54. Options (iii) and (iv) are the statements which are not true since in impurity defect there is a replacement of ions by the ions of another compound. By doing so, even though the electrical neutrality of the solid is maintained, a vacancy defect is caused due to creation of vacant sites in the ionic solid. This results into change in density of the solid. For example, if molten NaCl containing a little amount of SrCl2 is crystallised some of the sites of Na+ are occupied by Sr2+ .
Each Sr2+ replaces two Na+ ions and hence this affects density. Frenkel defect causes vacancy defect at its original site and an interstitial defect at its new location. Therefore, it does not change the density of the solid. Thus, the statement at option (iv) is also not true.
55. Which of the following statements are true about metals?
(i) Valence band overlaps with conduction band.
(ii) The gap between valence band and conduction band is negligible.
(iii) The gap between valence band and conduction band cannot be determined.
(iv) Valence band may remain partially filled.
55. (i), (ii) and (iv) is correct since gap between valence band and conduction band determines the conductivity of the metals. The electrical conduction of metals depend upon the type of valence band & also the gap between the valence band and conduction band while the valence band may remain partially filled.
56. Under the influence of electric field, which of the following statements is true about the movement of electrons and holes in a p-type semiconductor?
(i) Electron will move towards the positively charged plate through electron holes.
(ii) Holes will appear to be moving towards the negatively charged plate.
(iii) Both electrons and holes appear to move towards the positively charged plate.
(iv) Movement of electrons is not related to the movement of holes.
56. Options (i) and (ii) are correct since Si or Ge of group-14 are doped with electron deficient impurity i.e., group-13 elements like B, Al, or Ga containing only three valence electrons and hence is called as p-type semiconductors while the electron holes are created whenever an atom of such trivalent metal gets bonded with three of its neighbouring tetravalent silicon or germanium atoms.
The fourth valence electron of Ge or Si moves through the holes and under the influence of the electric field electrons move towards the positively charged plate through these holes. But it would appear as if the electron holes are positively charged and are moving towards the negatively charged plate.
57. Which of the following statements are true about semiconductors?
(i) Silicon doped with electron rich impurity is a p-type semiconductor.
(ii) Silicon doped with an electron rich impurity is an n-type semiconductor.
(iii) Delocalised electrons increase the conductivity of doped silicon.
(iv) An electron vacancy increases the conductivity of n-type semiconductor.
57. Options (ii) and (iii) are correct since silicon has 4 valence electrons and is doped with electron rich impurity is an n-type semiconductor due to extra electrons and hence the availability of the delocalised electrons increase the conductivity of this.
58. An excess of potassium ions makes KCl crystals appear violet or lilac in colour since ________.
(i) Some of the anionic sites are occupied by an unpaired electron.
(ii) Some of the anionic sites are occupied by a pair of electrons.
(iii) There are vacancies at some anionic sites.
(iv) F-centres are created which impart colour to the crystals.
58. Options (i) and (iv) are correct since an excess of K+ ions make KCl crystals appear violet due to the defect called metal excess defect . The anionic vacancies are found to be created due to the movement of potassium atoms to the surface of the crystal when KCl is heated in an atmosphere of potassium vapours and the Cl-ions then diffuse to the surface.
This happens due to the loss of the electron by the potassium atom to form K+ ions. In this case the electrons released diffuse to the crystal and occupy anionic sites being created. The anionic sites occupied by unpaired electrons are known as F-centres. These F-centres impart colour to the crystals for instance - yellow to NaCl and violet to KCl due to the excitation of these electrons by absorbing energy from the light falling on these crystals.
59. The number of tetrahedral voids per unit cell in NaCl crystal is ________.
(i) 4
(ii) 8
(iii) Twice the number of octahedral voids.
(iv) Four times the number of tetrahedral voids.
59. Options (ii) and (iii) are correct since the number of tetrahedral voids per unit cell in NaCl is calculated as-
Since NaCl crystal forms fcc lattice so,
The number of atoms (n) per unit cell = 4
So, the number of octahedral voids = n = 4
While the number of tetrahedral voids = 2n = 2*4=8
60. Amorphous solid can also be called ________.
(i) Pseudo solids
(ii) True solids
(iii) Super cooled liquids
(iv) Super cooled solids
60. Options (i) and (iii) are correct since the structure of the amorphous solids is similar to that of the liquids and hence they tend to flow like liquids, although very slowly. So, sometimes they are known as super cooled liquids or pseudo solids.
61. A perfect crystal of silicon (Fig. 1.1) is doped with some elements as given in the options. Which of these options show n-type semiconductors?
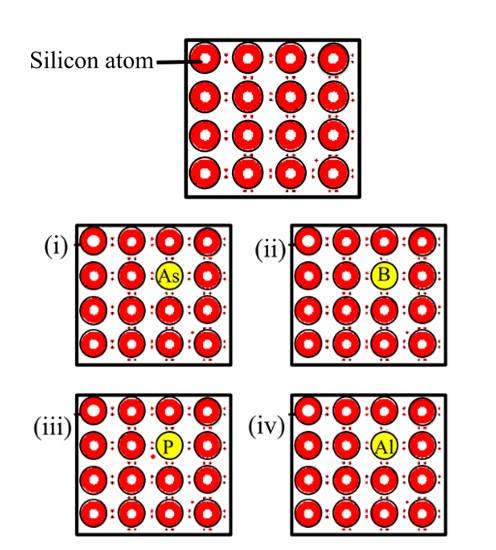
61. Options (i) and (iii) are correct since n-type semiconductors are prepared by doping silicon with pentavalent elements of group 15 (like P or As). Some of the lattice sites in silicon crystals are occupied by the fifth electron which is left over as extra electron and couldn't form a bond with the tetravalent Silicon and hence the images (i) and (iii) are showing this situation and hence representing n-type semiconductor.
62. Which of the following statements are correct?
(i) Ferrimagnetic substances lose ferrimagnetism on heating and become paramagnetic.
(ii) Ferrimagnetic substances do not lose ferrimagnetism on heating and remain ferrimagnetic.
(iii) Antiferromagnetic substances have domain structures similar to ferromagnetic substances and their magnetic moments are not cancelled by each other.
(iv) In ferromagnetic substances, all the domains get oriented in the direction of magnetic field and remain as such even after removing magnetic field.
62. Options (i) and (iv) are correct since these statements agrees with the ferrimagnetic and ferromagnetic nature of the substances respectively.
63. Which of the following features are not shown by quartz glass?
(i) This is a crystalline solid.
(ii) Refractive index is same in all the directions.
(iii) This has definite heat of fusion.
(iv) This is also called super cooled liquid.
63. Options (i) and (iii) are correct since quartz glass is an amorphous solid so, it does not exhibit a definite heat of fusion.
64. Which of the following cannot be regarded as molecular solid?
(i) SiC (Silicon carbide)
(ii) AlN
(iii) Diamond
(iv) I2
64. Options (i), (ii) and (iii) are correct since SiC, AlN, and diamond are not regarded as molecular solids, instead they come under network or covalent solids.
65. In which of the following arrangements octahedral voids are formed?
(i) Hcp
(ii) Bcc
(iii) Simple cubic
(iv) Fcc
65. Options (i) and (iv) are correct since when the tetrahedral void of the first layer and the tetrahedral void of the second layer align together, they form an octahedral void, and this is possible in case of hep and fcc arrangements.
In the following questions match the items given in Column I with the items given in Column II.
In some questions more than one item of Column I and Column II may match.
68. Match the defects given in Column I with the statements given.
|
Column I |
Column II |
|
(i) Simple vacancy defect |
(a) shown by non-ionic solids and increases density of the solid. |
|
(ii) Simple interstitial defect |
(b) shown by ionic solids and decreases density of the solid. |
|
(iii) Frenkel defect |
(c) shown by non-ionic solids and density of the solid decreases |
|
(iv) Schottky defect |
(d) shown by ionic solids and density of the solid remains the same |
68. (i) → (c); (ii) → (a); (iii) → (d); (iv) → (b)
69. Match the type of unit cell given in Column I with the features given in Column II.
|
Column I |
Column II |
|
(i) Primitive cubic unit cell |
(a) Each of the three perpendicular edges compulsorily have the different edge length i.e., a≠b≠c. |
|
(ii) Body centred cubic unit cell |
(b) Number of atoms per unit cell is one. |
|
(iii) Face centred cubic unit cell |
(c) Each of the three perpendicular edges compulsorily have the same edge length i.e., a = b = c |
|
(iv) End centred orthorhombic |
(d) In addition to the contribution from the unit cell to the corner atoms, the number of atoms present in a unit cell is one. |
|
(e) In addition to the contribution from the corner atoms the number of atoms present in a unit cell is three. |
69. (i) → (b); (ii) → (c); (iii) → (c); (iv) → (a)
70. Match the types of defects given in Column I with the statement given in Column II.
|
Column I |
Column II |
|
(i) Impurity defect |
(a) NaCl with anionic sites called F-centres |
|
(ii) Metal excess defect |
(b) FeO with Fe3+ |
|
(iii) Metal deficiency defect |
(c) NaCl with Sr2+ and some cationic sites vacant |
70. (i) → (c); (ii) → (a); (iii) → (b)
71. Match the items given in Column I with the items given in Column II.
|
Column I |
Column II |
|
(i) Mg in solid state |
(a) p-Type semiconductor |
|
(ii) MgCl2in molten state |
(b) n-Type semiconductor |
|
(iii) Silicon with phosphorus |
(c) Electrolytic conductors |
|
(iv) Germanium with boron |
(d) Electronic conductors |
71. (i) → (d); (ii) → (c); (iii) → (b); (iv) → (a)
72. Match the type of packing given in Column I with the items given in Column II.
|
Column I |
Column II |
|
(i) Square close packing in two dimensions. |
(a) Triangular voids |
|
(ii) Hexagonal close packing two dimensions. |
(b) Pattern of spheres is repeated in every fourth layer |
|
(iii) Hexagonal close packing in three dimensions. |
(c) Coordination number = 4 |
|
(iv) Cubic close packing in three dimensions. |
(d) Pattern of sphere is repeated in alternate layers |
72. (i) → (c); (ii) → (a); (iii) → (d); (iv) → (b)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(i) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(ii) Assertion and reason both are correct statements, but reason is not correct explanation for assertion.
(iii) Assertion is correct statement, but reason is wrong statement.
(iv) Assertion is wrong statement, but reason is correct statement.
73. Assertion: The total number of atoms present in a simple cubic unit cell is one.
Reason: Simple cubic unit cell has atoms at its corners, each of which is shared between eight adjacent unit cells.
73. Option (i) Assertion and reason both are correct statements and reason is correct explanation for assertion is the answer since in simple cubic crystal unit cell, the total number of atoms are given as - 8 * 18=1
74. Assertion: Graphite is a good conductor of electricity however diamond belongs to the category of insulators.
Reason: Graphite is soft in nature on the other hand diamond is very hard and brittle.
74. Option (ii) Assertion and reason both are correct statements, but reason is not correct explanation for assertion is the answer since in graphite carbon atoms are arranged in different layers and each atom is covalently bonded to three of its neighbouring atoms in the same layer due to which the fourth valence electron of each atom, present between the different layers is free to move and this makes graphite a good conductor of electricity.
As different layers can slide over each other which makes it soft. while in case of diamond C atom is bonded covalently with their adjacent atoms throughout the crystal. Thus, all the four valencies are satisfied. Covalent bonds are strong and directional in nature ; therefore, atoms are held very strongly at their positions. This is why diamond is hard and has a very high melting point but decomposes before melting, also acting as an insulator.
75. Assertion: Total number of octahedral voids present in unit cell of cubic close packing including the one that is present at the body centre, is four.
Reason: Besides the body centre there is one octahedral void present at the centre of each of the six faces of the unit cell and each of which is shared between two adjacent unit cells.
75. Option (iii) Assertion is the correct statement, but the reason is wrong is the answer since besides the body centre there is one octahedral void at the centre of each 12 edges which is shared between four adjacent unit cells. Thus, Octahedral voids present at the body centre of the cube = 1
12 octahedral voids are located at each edge and shared between four-unit cells = 12 * = 3
Total number of octahedral voids = 4.
JEE Mains 2022
JEE Mains 2022
Commonly asked questions
Match List I with List II:
Choose the correct answer form the options given below:
BrF5 - Square pyramid
[CrF6]3- - Octahedral
O3 - Bent
PCl5 - Trigonal Bipyramid
Match List I with List II:
|
List-I (Processes/Reactions) |
List-II (Catalyst) |
|
(A) 2SO2(g) + O2(g) -->2SO3(g) |
(I) Fe(s) |
|
(B) 4NH3(g) + 5O2(g)--> 4NO(g) + 6H2O(g) |
(II) Pt(s) – Rh(s) |
|
(C) N2(g) + 3H2(g) --> 2NH3(g) |
(III) V2O5 |
|
(D) Vegetable oil (I) + H2 --> Vegetable ghee(s) |
(IV) Ni(S) |
Choose the correct answer from the options given below:
ostwald’s process
Vegetable oil (l) + H2 vegetable ghee; Hydrogenation
Given two statements below:
Statement I : In Cl2 molecules the covalent radius is double of the atomic radius of chlorine.
Statement II : Radius of anionic species is always greater than their parent atomic radius.
Choose the most appropriate answer from options given below:
Ionic radius of Cl- = 167 pm
Covalne radius of Cl = 99 pm
Refining using liquation method is the most suitable for metals with:
Liquation process is used for purification of that metal, whose melting point is lesser then that of impurities.
Which of the following can be used to prevent the decomposition of H2SO4?
Urea acts as negative catalyst for decomposition of H2O2.
Reaction of BeCl2 with LiAlH4 gives:
(A) AlCl3
(B) BeH2
(C) LiH
(D) LiCl
(E) BeAlH4
Choose the correct answer from options given below:
Borazine, also known as inorganic benzene, can be prepared by the reaction of 3-equivalents of “X” with 6-equivalents of “Y”. ‘X’ and “Y”, respectively are:
Which of the given reactions is not an example of disproportionation reaction?
It is reduction process, while rest are examples of disproportionation reaction.
The dark purple colour KMnO4 disappears in the titration with oxalic acid in acidic medium. The overall change in the oxidation number of manganese in the reaction is:
change in oxidation number of Mn = 7 – 2 = 5
+ CH4 --> A + B
A and B in the above atmosphere reaction step are:
Which technique among the following is most appropriate in separation of a mixture of 100mg of p-nitrophenol acid and picric acid?
Solvent polarity is related to Rf value of nitro compounds
100 mg P-nitrophenol and picric acid have different Rf value on silica gel plate.
Preparative TLC is best to separate 100 mg of P-nitro phenol and picric acid.
Which of the following compounds in not aromatic?
Both indicated hydrogen atoms repel each other so due steric hinderance the given compound becomes non planar. It is non-aromatic.
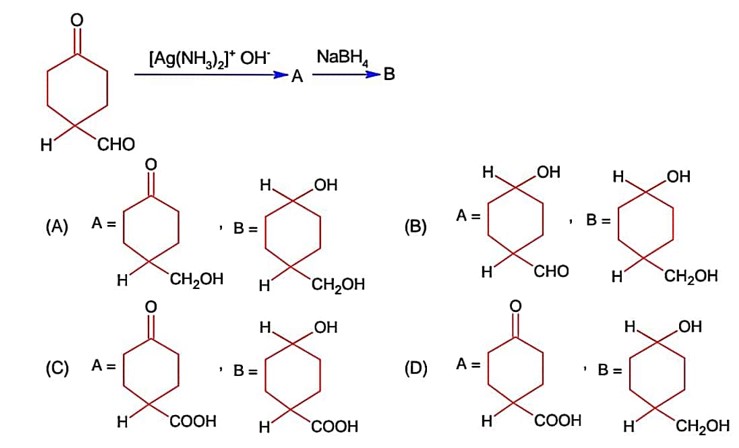
The products formed in the following reaction, A and B are
Kindly consider the following figure
Which reactant will give the following alcohol on reaction with one mole of phenyl magnesium bromide (PhMgBr) followed by acidic hydrosis?
Kindly consider the following figure
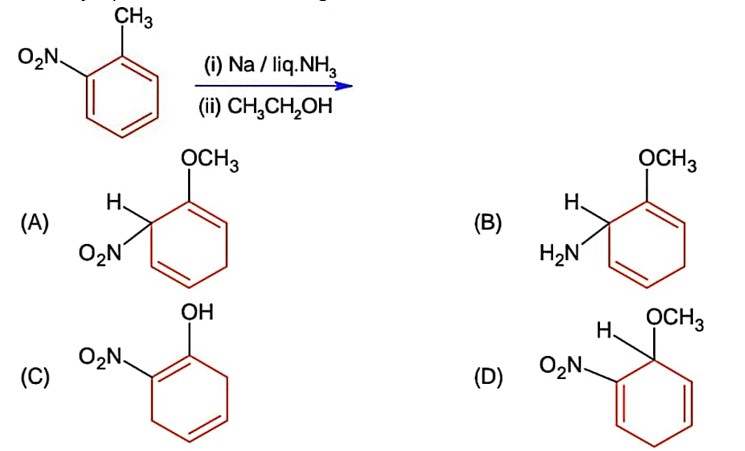
The major product of the following reaction is
Birch reduction takes place of carbon connected with electron withdrawing group (-NO2)
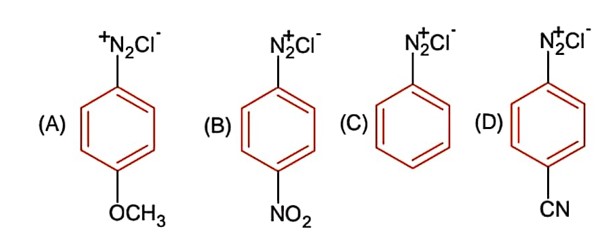
The correct stability order of the following diazonium salt is
Electron donating group increases stability of diazonium salt. Whereas electron with drawing group decrease it.
-OCH3 (+M), -NO2 & -CN (-M)
Stearic acid and polyethylene glycol react to form which one of the following soap/s etergents?
Kindly consider the following figure
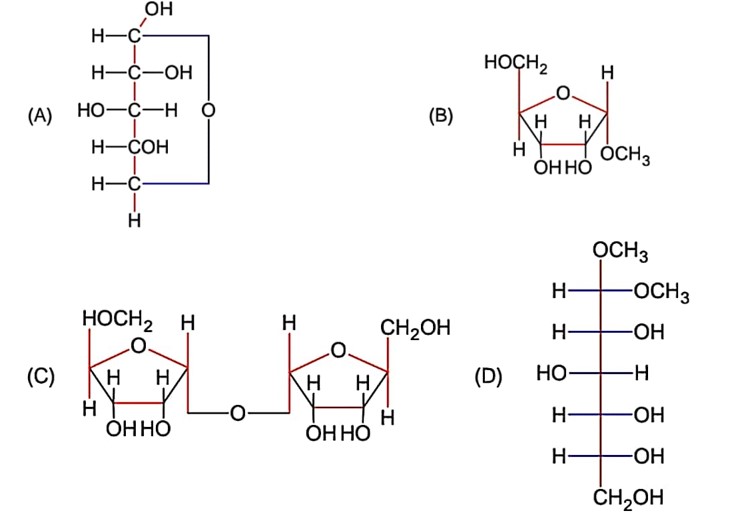
Which one of the following is a reducing sugar?
Reducing sugar must have hemi-acetal group and option (A) has that group.
Given below are two statements : one is labeled as Assertion (A) and the other is labeled as
Reason (R).
Assertion (A): Experimental reaction of CH3Cl with aniline and anhydrous AlCl3 does not give O and P-methylaniline.
Reason (R) : The -NH2 groups of aniline becomes deactivating because of salt formation with anhydrous AlCl3 and hence yields m-methyl aniline as the product.
In the light of the above statements, choose the most appropriate answer from the options given below:
Aniline makes adduct with AlCl3 and thus gets deactivated for electrophilic aromatic substitution and do not react with CH3-Cl
Chlorophyll extracted form the crushed green leaves was dissolved in water to make 2 L solution of Mg of concentration 48 ppm. The number of atoms of Mg in this solution is x × 1020 atoms. The value of x is _____________. (Nearest Integer)
(Given : Atomic mass of Mg is 24 g mol-1; NA = 6.02 × 1023 mol-1)
Mass of solute = 48 * 2 * 10-3 gram
Atoms of Mg = 24.08 * 1020
A mixture of hydrogen and oxygen contains 40% hydrogen by mass when the pressure is 2.2 bar. The partial pressure of hydrogen is __________ bar. (Nearest Integer)
= 0.914
The wavelength of an electron and a neutron will become equal when the velocity of the electron is x times the velocity of neutron. The value of x is ___________. (Nearest Integer)
(Mass of electron is 9.1 × 10-31kg and mass of neutron is 1.6 × 10-27kg)
Xe = Xn
MeVe = MnVn
2.4g coal is burnt in a bomb calorimeter in excess of oxygen at 298 K and 1 atm pressure. The temperature of the calorimeter rises from 298 K to 300 K. The enthalpy change during the combustion of coal is -x kJ mol-1. The value of x is ____________.
(Given : Heat capacity of bomb calorimeter 20.0 kJ K-1. Assume coal to be pure carbon)
Number of moles of C =
When 800mL of 0.5 M nitric acid is heated in a beaker, its volume is reduced to half and 11.5g of nitric acid is evaporated. The molarity of the remaining nitric acid solution is x × 10-2M. (Nearest Integer)
(Molar mas of nitric acid is 63g mol-1)
Remaining volume of solution = 400 ml
Mass of HNO3 = 25.2 – 11.5 = 13.7
Molarity = = 0.54 M = 54 × 10-2 M
At 298 K, the equilibrium constant is 2 × 1015 for the reaction:
Cu(s) + 2Ag+(aq) Cu2+(aq) + 2Ag(s)
The equilibrium constant for the reaction
+ Ag(s) + Ag+ (aq)
is x × 10-8. The value of x is _____________. (Nearest Integer)
The amount of charge in F (Faraday) required to obtain one mole of iron from Fe3O4 is __________. (Nearest Integer)
Fe3O4 + 8H+ + 8e -> 3Fe + 4H2O
For a reaction A--> 2B + C the half lives are 100s and 50s when the concentration of reactant A is 0.5 and 1.0 mol L-1 respectively. The order of the reaction is _____________. (Nearest (Integer)
where n = order of reaction.
The difference between spin only magnetic moment value of is _______________.
Number of unpaired electrons = 3
Number of unpaired electrons = 3
In the presence of sunlight, benzene reacts with Cl2 to give product, X. The number of hydrogens in X is_______________.
Kindly consider the following figure
Chemistry NCERT Exemplar Solutions Class 12th Chapter One Exam